Neuromodulation May Benefit Patients With Varying Psychiatric Illnesses
Abstract
Neuromodulation treatments work in a “top-down” manner, altering the transmission of information at the level of brain networks, which feeds down to the level of the individual synapse.

While many are accustomed to thinking of communication in the brain as being driven by neurotransmitters, the brain networks that regulate mood, thought, and circadian rhythms are bound together by both electrical and chemical neurotransmission.
The brain utilizes more energy per ounce than any other organ in the body, and most of this energy is devoted to creating and sustaining the electrical charge of neurons. Electrical firing of neurons creates functional networks in the brain and facilitates the release of serotonin, norepinephrine, and other neurotransmitters. Interestingly, extracellular levels of these neurotransmitters also regulate the rate and pattern of electrical firing of neurons and the frequency of rhythmic brain oscillations. Electrical and chemical neurotransmission are in essence two sides of the same coin, creating and sustaining functional brain networks (1, 2).
Thus it is not surprising that neuromodulation—technologies that apply magnetic or electrical energy to the brain to alter neurotransmission—have been found to produce therapeutic effects similar to those of medication.
Repetitive transcranial magnetic stimulation (rTMS), one of the newest treatments for major depressive disorder (MDD), is one such technique. It utilizes electromagnetic induction to create small currents in key mood-regulating areas of the prefrontal cortex (2).
The most common rTMS treatment target is the left dorsolateral prefrontal cortex (DLPFC). Patients treated with at least 30 sessions of high-frequency (10 Hz) rTMS in this region have been reported to have response and remission rates of up to 58 percent and 37.1 percent, respectively, when combined with antidepressant medication (3).
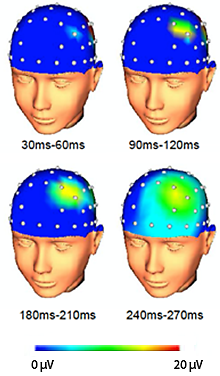
Within 30 ms of TMS pulses, an area of increased cortical activity emerges at the site of stimulation (indicated by a focus of red in the F3 electrode overlying left DLPFC). Over the next 200 ms, the area of increased activity can be seen spreading to neighboring areas of cortex.
The benefits of rTMS treatment for MDD can be sustained for a year in up to 70 percent of patients (4) and are similar to those seen in patients taking medication alone (5).
rTMS is not electroconvulsive therapy (ECT)—one of the first neuromodulation techniques introduced nearly 80 years ago, which involves broad delivery of depolarizing current substantial enough to induce a seizure. rTMS uses magnetic induction to depolarize neurons and cause firing but only in a focal area under the stimulating magnet. Other neuromodulation techniques include trigeminal nerve stimulation (TNS), which introduces a subthreshold current into the brain through a superficial branch of the trigeminal nerve, and deep brain stimulation (DBS), which introduces very small currents directly into mood-regulating areas deep in the brain such as the anterior cingulate cortex.
Despite the notable differences in these techniques, all have been reported to have antidepressant efficacy (7).
What’s Behind Neuromodulation’s Antidepressant Effects?
We are accustomed to thinking of pharmacologic treatments that penetrate the brain broadly as antidepressant treatments. How can the application of energy at such disparate levels and diverse manners have similar antidepressant efficacy?
The action of neuromodulation treatments can best be understood from the standpoint of network-based treatments of depression. These treatments are applied to brain regions that constitute “hubs” of mood-regulating networks of the brain (2). For example, rTMS stimulation of any one of these hubs does not remain local, but increases cortical excitation that spreads over the surface of the brain within milliseconds (see figure on left above). At the same time, rTMS administered to the left DLPFC rapidly induces blood flow changes throughout the limbic system in the thalamus, caudate, and other subcortical and cortical areas with connections to the site of stimulation (8). Similarly, TNS changes blood flow in anterior cingulate cortex, the inferior frontal gyrus, and medial and middle frontal gyri including the DLPFC (7).
There are many levels at which antidepressant treatments may work: within an individual cell, at the level of cell-to-cell communication (i.e., chemical or electrical synapses), or at the level of communication among larger functional groupings of cells (i.e., brain circuits or networks).
Neuromodulation Acts Along a Continuum of Structures, Processes
The syndrome of MDD can be understood along a continuum of structures and processes ranging from the level of a single cell to ensembles of neurons in brain networks. These structures and processes can be grouped into three general levels of biological complexity: the level of the single cell, the level of cell-to-cell communication, and the level of cell networks (see related figure here). These three levels are not entirely distinct.
Processes at any level along this continuum exert physiologic effects in a bidirectional manner: intracellular processes or intercellular communication transmit their effects “bottom-up” to affect brain functional networks, whereas processes that directly modulate function in brain networks or microcircuits transmit their effects “top-down” to influence cellular processes or intercellular communication (see related figure here). Evidence indicates that, in addition to synaptic neurotransmission, neuroelectric communication plays a central role in mediating top-down and bottom-up effects across multiple levels of biological complexity in the brain.
Neuromodulation and medication treatments for MDD are shown graphically in relation to their hypothesized level of action along this continuum here. Neuromodulation treatments (such as rTMS) modulate oscillations in higher-order brain networks (arrow at top left), whereas medication treatments affect synaptic or neuroelectric neurotransmission at the level of cell-to-cell communication (arrow at middle left). Modulation of neuronal firing rates and patterns and oscillatory synchrony may represent mechanisms through which treatment effects are transduced across multiple levels of physiologic action to increase neuroplasticity. Adapted from Leuchter et al., 2015 (2).
Antidepressant medications can be conceptualized as working “bottom-up” from the level of the synapse, changing transmission between individual neurons that then feeds up to the level of circuits, networks, and the brain as a whole. Conversely, neuromodulation treatments work in a “top-down” manner, altering the transmission of information at the level of brain networks that then feeds down to the level of the individual synapse.
While medications and neuromodulation treatments work at different levels of biological complexity, they presumably both feed down to affect neuronal processes such as cell metabolism and gene transcription. Neuromodulation and medication treatments of MDD may in fact be synergistic, interacting across different levels of biological complexity to enhance the efficacy of the treatments (2). Such synergy could explain why patients who do not appear to respond during a course of rTMS treatment may experience a reduction in depressive symptoms during later courses of medication treatment (4).
The Future of Neuromodulation
There is a great deal that remains unknown regarding the efficacy of neuromodulation treatments for MDD.
There are a number of possible treatment targets for rTMS, including left DLPFC, right DLPFC, as well as dorsomedial prefrontal cortex. There are also a number of different frequencies and patterns of stimulation (“pulse sequences”) that appear to be effective ranging from 1 Hz to 10 Hz and even higher (so-called “theta burst” stimulation) (9). The parameter space for adjusting these factors—one or more site(s) of simulation, and frequency and pattern of stimulation—is huge. Methods such as electroencephalographic (EEG) recording during stimulation to determine how stimulation alters brain network function may offer insights into how best to direct TMS treatment for each individual (10).
Finally, there are multiple neuromodulation technologies that may be effective for MDD that have not been fully explored and/or approved by the Food and Drug Administration. Such technologies include repetitive transcranial direct current stimulation (tDCS) or transcranial alternating current stimulation (tACS), which utilize small batteries and electrodes attached to the scalp, and synchronized transcranial magnetic stimulation (sTMS), which utilizes rotating neodymium magnets to impart low intensity sinusoidal waveform stimulation (6-7). Some of these technologies have been determined to pose no significant risk to the patient and, once efficacy is more clearly established, may even be available for patients to use at home.
Neuromodulation treatments also appear to have efficacy beyond the treatment of MDD; for example, studies suggest that rTMS can also be used for treatment of obsessive-compulsive disorder, posttraumatic stress disorder, fibromyalgia and other chronic pain conditions, auditory hallucinations, and other neuropsychiatric illnesses.
As the evidence base for neuromodulation treatments continues to grow, diagnostic tests will increasingly be used by clinicians to guide the selection of the most effective neuromodulation procedure or to adjust treatment settings for each patient. In addition to prescribing medications, psychiatrists may one day find themselves performing office-based brain stimulation procedures and prescribing devices to be used at home. ■
1. Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88(1):220-35.
2. Leuchter AF, Hunter AM, Krantz DE, Cook IA. Rhythms and blues: modulation of oscillatory synchrony and the mechanism of action of antidepressant treatments. Ann N Y Acad Sci. 2015;1344:78-91.
3. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-96.
4. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-401.
5. Demitrack MA, Thase ME. Clinical significance of transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant depression: synthesis of recent data. Psychopharmacol Bull. 2009;42(2):5-38.
6. Neuromodulation Division at the UCLA Semel Institute. http://neuromodulation.ucla.edu/
7. Cook IA, Espinoza R, Leuchter AF. Neuromodulation for depression: invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation). Neurosurg Clin N Am. 2014;25:103-16.
8. Hanlon CA, Canterberry M, Taylor JJ, et al. Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One. 2013;8(7):e67917.
9. Downar J, Daskalakis ZJ. New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 2013;6(3):231-40.
10. Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci Biobehav Rev. 2016;64:175-184.



