Antipsychotics’ Diabetes Risk Prompts Call for Better Assessment
A new consensus statement confirms that the risk of significant treatment-emergent changes in glucose metabolism associated with second-generation antipsychotics (SGAs) requires careful assessment and continued monitoring. In addition, the statement says, the risk of metabolic abnormalities differs among the six available medications.
Darrel Regier, M.D., Ph.D., head of APA’s Office of Research and the American Psychiatric Institute for Research and Education, noted that the consensus panel statement, while consistent with emerging research, is not an official position statement of APA. Regier said that the Association’s Council on Research has a work group that is developing recommendations for an APA position statement that will go into detail on the mechanisms affecting lipid and glucose metabolism and will include a discussion of treatment implications.
The eight-member panel heard presentations from 14 experts in psychiatry, obesity, and diabetes, as well as presentations from the Food and Drug Administration (FDA) and representatives of AstraZeneca, Bristol-Myers Squibb, Janssen, Lilly, and Pfizer, all of which market SGAs. The panel said it also reviewed the majority of the known peer-reviewed clinical literature on the issue as well as pertinent animal studies.
The panel’s deliberations were expected by many to be controversial from the start.
Last September the FDA required the manufacturers of all six second-generation drugs to relabel their products, adding common language stating that the drugs carry risk of metabolic side effects, including changes in glucose metabolism and lipid metabolism. Significantly, without directly saying so, the FDA appeared to put all of the six drugs on equal footing with regard to their risk of treatment-emergent diabetes (Psychiatric News, October 17, 2003).
Clinical evidence, however, has long suggested otherwise, and the panel’s statement dutifully notes this issue. (In fact, Pfizer recently submitted a supplemental new drug application, based on newly analyzed postmarketing data on ziprasidone. The application requests the FDA to approve wording for the Geodon label that in part acknowledges its apparent lack of metabolic side effect.)
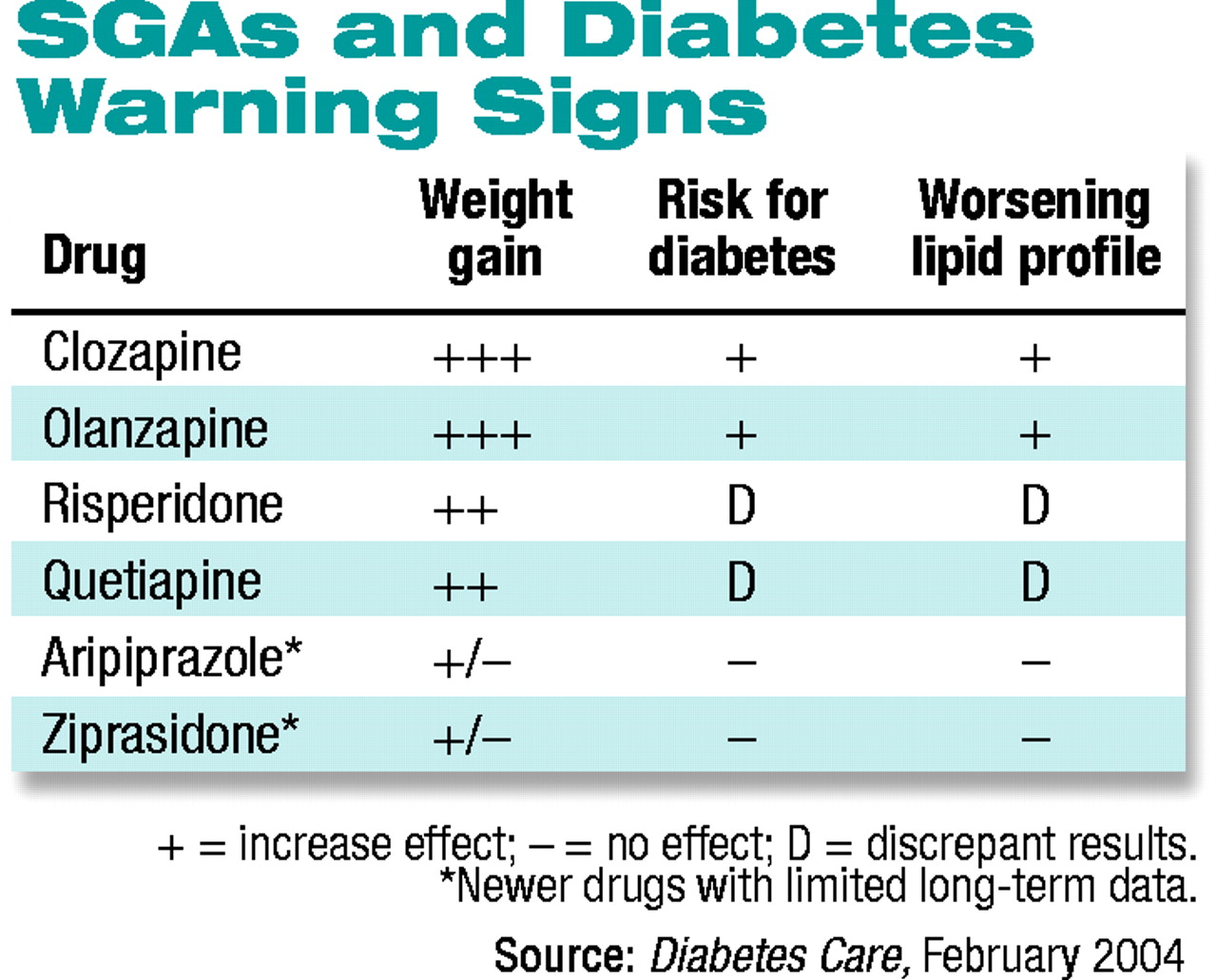
“The six currently available SGAs vary in their efficacy, formulation, biochemistry, receptor binding, and side-effect profiles,” the consensus statement says. It goes on to say that the prevalence of both diabetes and obesity appear to be 1.5 to 2.0 times higher in patients with schizophrenia and affective disorders than in the general population. However, “whether this is a function of the illness itself versus its treatment is unknown.”
The panel links the risk of treatment-emergent dysfunction in glucose metabolism to the antipsychotic medications’ propensity to cause weight gain. Because the liability for weight gain varies among the six drugs, the risk for diabetes also varies between among them, the statement concludes.
“Despite limitations in study design, the data consistently show an increased risk for diabetes in patients treated with clozapine [Clozaril] or olanzapine [Zyprexa] compared with patients not receiving treatment with first-generation antipsychotics or other second-generation antipsychotics.”
The risks associated with risperidone (Risperdal) and quetiapine (Seroquel) are “less clear,” the statement says, noting that some studies show increased risk for diabetes associated with these two drugs, while other studies do not.
Lastly, the two most recently introduced medications—ziprasidone (Geodon) and aripiprazole (Abilify)—“have relatively limited epidemiological data,” but clinical trial data indicate little or no risk for diabetes.
The differentiation of the six medications—which conflicts with the FDA’s earlier stance—drew an immediate response from Zyprexa-maker Eli Lilly and Co. In a press release, the company blasted the consensus statement, saying the company “does not agree with the controversial conclusion. . . , which states that second-generation antipsychotics differ in their diabetes risk profiles.” Lilly noted the FDA’s own warning language says that “precise risk estimates for hyperglycemia-related adverse events in patients treated with atypical antipsychotics are not available.”
Lilly said, however, that the company agrees with the majority of the consensus statement, including the call for baseline screening and follow-up monitoring for patients taking SGAs.
The consensus panel notes that despite the adverse effects associated with these medications, great clinical benefit has been derived from them.
However, “the risks of obesity, diabetes, and dyslipidemia have considerable clinical implications in this patient population and should influence drug choice.” A careful risk-benefit analysis must be done for each patient, the statement says, and both physicians and patients would benefit from the continued availability “of a broad array of therapeutic agents.”
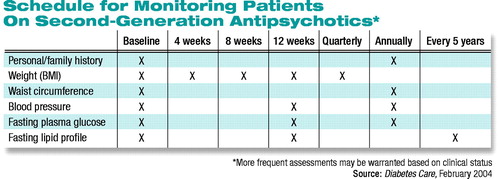 The statement urges clinicians who prescribe these agents to assess and monitor key indicators of individual patients’ baseline risk for the development of metabolic adverse effects. A thorough family history must be taken, and patients’ weight, waist circumference, and blood pressure recorded. In addition, baseline values must be established for the patient’s fasting plasma-glucose level and fasting lipid profile (see table above).
The statement urges clinicians who prescribe these agents to assess and monitor key indicators of individual patients’ baseline risk for the development of metabolic adverse effects. A thorough family history must be taken, and patients’ weight, waist circumference, and blood pressure recorded. In addition, baseline values must be established for the patient’s fasting plasma-glucose level and fasting lipid profile (see table above).
“These assessments,” the statement says, “can determine if the patient is overweight (BMI 25.0-29.9) or obese (BMI 30.0), has pre-diabetes (fasting plasma glucose of 100-125 mg/dl) or diabetes (fasting plasma glucose 126 mg/dl), hypertension (blood pressure >140/90 mmHg), or dyslipidemia.”
If any of the above are present, the statement stresses, appropriate treatment must be initiated, including referral to specialists.
The statement includes a monitoring schedule for periodic assessment of glucose, lipid, and blood pressure levels over the long term. If a patient gains more than 5 percent of his or her initial weight while taking an antipsychotic, the statement strongly urges clinicians to consider switching the patient to a medication with less of a weight-gaining liability. A switch should also be considered if a patient develops treatment-emergent worsening of glucose or lipid levels while taking an SGA.
In addition, the statement calls for significantly increased research efforts to help answer key questions, such as, Are there early treatment factors that could predict developing problems? If so, what are they?
The consensus statement is posted online at http://care.diabetesjournals.org/cgi/content/full/27/2/596. ▪
Diabetes Care 2004 27 596



