Depressed Youth Respond Best to Combination Therapy
Combining medication and psychotherapy provides adolescents with depression the treatment advantages of both a sprint and a marathon.
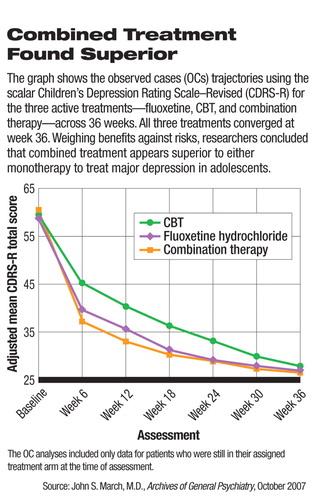
The combination of the antidepressant medication fluoxetine (Prozac) and cognitive-behavioral therapy (CBT) appears more effective than either strategy alone for the long-term treatment of adolescents with depression, according to the latest report from the Treatment for Adolescents With Depression Study (TADS) in the October Archives of General Psychiatry.
The report lengthens observations in the TADS from the 12 weeks reported earlier this year (Psychiatric News, March 2) to 36 weeks.
The benefit of combination therapy derives from the swifter initial action of the drug linked with the longer term effects of CBT, said lead author John March, M.D., M.P.H., professor and chief of child and adolescent psychiatry in the Department of Psychiatry and Behavioral Science at Duke University.
All three treatments proved equally successful at the end of the trial, but the response rate at that point is not the only standard to use, said March in an interview with Psychiatric News.
“There are a lot of things we can do to get a patient better, but faster is important too,” he said. “Three months in the life of a depressed kid is a long time.”
The longer trial period is a more clinically relevant time frame, said Stan Kutcher, M.D., the Sun Life Financial Chair in Adolescent Mental Health in the Department of Psychiatry at Dalhousie University in Halifax, Nova Scotia.
“Nobody treats depression for just 12 weeks,” Kutcher told Psychiatric News. “This is the best information we have to date about treating this disorder in young people.”
The TADS study, funded by the National Institute of Mental Health (NIMH) in 1999, is part of ongoing research seeking to improve the treatment of depression in youth. According to the researchers, major depressive disorder affects approximately 5 percent of adolescents.
The researchers randomly assigned 327 patients aged 12 to 17 with a DSM-IV diagnosis of major depression to one of the three treatment conditions: combination therapy (n=107), fluoxetine therapy (n=109), or CBT (n=111).
Fluoxetine was initially prescribed at a dosage of 10 mgs a day and then titrated in response to whether patients experienced positive reactions to treatment or underwent adverse effects. Patients receiving CBT had 15 one-hour sessions during the first 12 weeks; thereafter, therapy was usually less frequent, dependent on how the patients responded to treatment.
Positive responders were those who showed as “very much improved” or “much improved” on the Clinical Global Impressions—Improvement scale (CGI-I), which requires the clinician to rate how much the patient's illness has improved or worsened relative to baseline.
According to the researchers, 73 percent of patients receiving combination therapy, 62 percent of those receiving fluoxetine only, and 48 percent of those receiving CBT only responded to treatment after the initial 12 weeks. At the end of 36 weeks, 243 (74 percent) of the patients remained in the study. Positive response rates at that time were 86 percent for combination therapy, 81 percent for fluoxetine, and 81 percent for CBT.
CBT Response Increased Dramatically
However, the response to CBT alone increased from 48 percent to 65 percent by week 18, while fluoxetine therapy response increased only from 65 percent to 69 percent over the same period.
“The data show that CBT caught up with fluoxetine therapy by weeks 18 to 24 and to combination therapy by weeks 30 to 36,” the researchers said.
“Starting patients on fluoxetine improved functioning and lowered illness burden quickly, while adding CBT seems to have had an additional effect on modulating suicide,” said Kutcher, who was not involved in the trial.
The TADS findings were broadly generalizable, said the authors, because the study population included both sexes, older and younger adolescents, minority representation proportionate to the U.S. population, and those from varied socioeconomic backgrounds.
“We designed the trial to represent what you'd want in clinical practice,” said March. “Patients were treated by real psychiatrists and psychologists who paid attention to the details of clinical practice, not just the protocols.”
Kutcher, who has also conducted clinical trials related to adolescent depression, was impressed by the study's ability to retain patients. Patient retention is often difficult in clinical trials, especially in those in which psychotropics are being used.
That retention, said March, was due to the effects of therapy as well as having plans in place for expectable contingencies.
Many Had Suicidal Ideation
About 30 percent of study patients met criteria for clinically significant suicidal ideation at baseline, based on the Suicidal Ideation Questionnaire-Junior High School Version (SIQ-Jr). Despite“ aggressive” treatment over 36 weeks, there were suicidal events (ideation, an attempt, or preparatory action) in 10 percent of patients, nearly 70 percent of them occurring in the first 12 weeks—although not evenly distributed across trial arms.
“There was twice the risk of suicidal events in the fluoxetine-only group (16 patients), compared with the CBT (seven patients) or combination (nine patients) groups,” said March. There were no completed suicides in the TADS.
“TADS replicated the Food and Drug Administration (FDA) findings on the suicidal-event rate using fluoxetine alone,” said March. An FDA meta-analysis was used as the basis for the initial black-box label warning regarding possible suicidality in youngsters taking antidepressants.
After considering the benefits and risks of the two treatments as monotherapies, the authors concluded that fluoxetine alone or in combination with CBT accelerated improvement of depression compared with CBT alone and that adding CBT to fluoxetine therapy minimized suicidal ideation and treatment-emergent suicidal events.
The TADS team will complete a full year of naturalistic follow-up, with some patients continuing treatment and some not. Grants from NIMH and the National Institute on Drug Abuse will also allow them to track their subjects for five years to record functional outcomes as they transition to college, young adulthood, and the workplace, said March.
TADS is a major contribution to guiding current practice, although only future research can tell if a different SSRI or another form of psychotherapy would give similar results, Kutcher said.
“We now have a safe and effective treatment for a very severe disorder,” he said. “Now providers have to ask what is our moral and social responsibility to children, given that probably less than 30 percent of children who need help are getting it.”
An abstract of “The Treatment for Adolescents With Depression Study (TADS)” is posted at<http://archpsyc.ama-assn.org/cgi/content/short/64/10/1132>.▪



