Lithium Bests Valproate in Preventing Relapse
Abstract
Lithium and valproate combination therapy is significantly more effective than valproate monotherapy in preventing manic relapse in patients with bipolar disorder in long-term therapy, but the combination did not beat lithium monotherapy. These findings come from a 10-year clinical trial conducted by researchers from four countries on both sides of the Atlantic.
The trial, known as Bipolar Affective disorder: Lithium/ANtiConvulsant Evaluation, or BALANCE for short, began in 2001 and was conducted at 41 sites in the United States, United Kingdom, France, and Italy. The findings were published in the January 30 The Lancet.
After demonstrating that they could tolerate both lithium and valproate during a short run-in period, 330 patients with a diagnosis of bipolar I disorder were randomized in equal proportions to one of three treatments: lithium carbonate only, sodium divalproex only, or the combination of both.
Patients were followed for up to two years and evaluated primarily for time to first relapse, defined as the emergence of a new mood episode that required new interventions, including boosting the medication dose, switching to a different drug, or hospitalization.
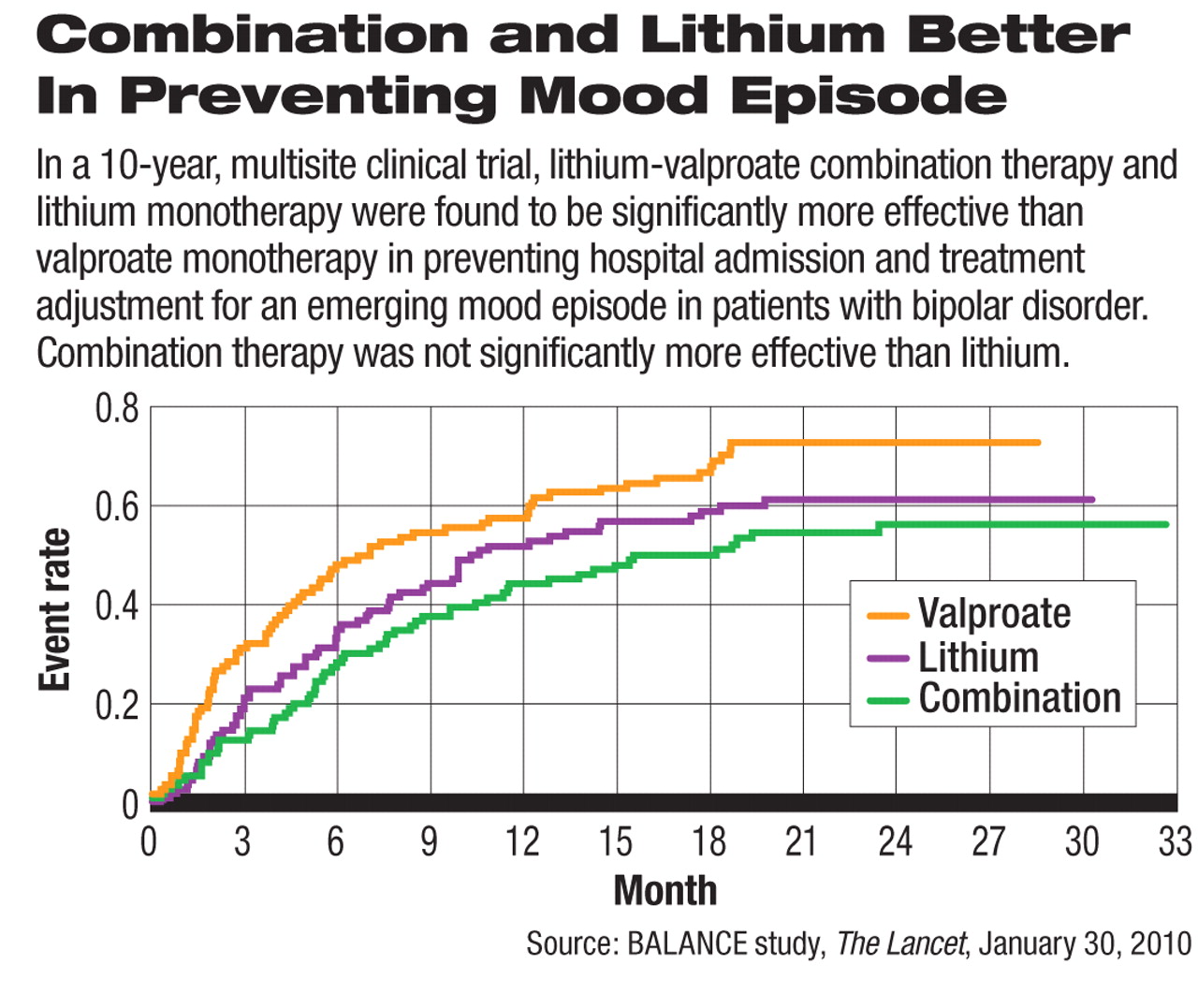
Based on statistical analysis, the risk of relapse was significantly lower for combination therapy compared with valproate monotherapy but not significantly different from lithium therapy. In addition, the risk of relapse was significantly lower for lithium monotherapy compared with valproate therapy.
Compared with valproate, combination therapy's advantage was the largest in preventing a manic relapse, while the advantage of lithium was most apparent in preventing a depressive relapse.
“BALANCE could neither confirm nor refute a benefit of combination therapy compared with lithium monotherapy,” the authors emphasized. They noted, however, that the therapeutic effect of adding lithium to valproate is “unequivocal and substantial.”
The study also demonstrated the long-term reality of bipolar disorder treatment. Over half of study participants in every treatment group had a relapse during the two-year follow-up period: 54 percent in the combination group, 59 percent in the lithium group, and 69 percent in the valproate group.
The proportion of patients who needed to be hospitalized was 15 percent in the combination group, 20 percent in the lithium group, and 23 percent in the valproate group. The proportion of patients who required new treatment for a manic episode ranged from 27 percent to 45 percent, while 35 percent to 45 percent of patients required new treatment for a depressive episode.
The trial was funded primarily by the Stanley Medical Research Institute, and the French trials were funded by Sanofi-Aventis.
In an accompanying editorial, Rasmus Licht from Aarhus University Hospital in Denmark commented that BALANCE is the first three-way comparative trial on long-term effectiveness of bipolar treatment that includes a combination treatment group. Although the knowledge gained from this study is extremely clinically relevant, pharmaceutical companies have little incentive to test whether combination therapy is better than monotherapy, he noted.
An abstract of “Lithium Plus Valproate Combination Therapy Versus Monotherapy for Relapse Prevention in Bipolar I Disorder (BALANCE): A Randomised Open-Label Trial” is posted at <www.thelancet.com/journals/lancet/article/PIIS0140-6736%2809%2961828-6/abstract>.



