Evidence Builds for Efficacy of Deep Brain Stimulation
Abstract
Seven years ago, neurologist Helen Mayberg, M.D., and her colleagues reported something rather remarkable—that six subjects with treatment-resistant unipolar depression seemed to benefit from electrical stimulation of the subcallosal cingulate region of the brain.
“When we started, no one knew if it would work, or if it did, how long improved symptoms might last,” Mayberg recalled during a recent interview.
Four years ago, she and her colleagues reported more encouraging findings along these lines—this time in an expanded sample of 20 subjects with treatment-resistant unipolar depression (Psychiatric News, September 5, 2008). Then last year came more—again in the same 20 subjects, but over a longer follow-up period (Psychiatric News, May 20, 2011).
And now comes still more news from Mayberg and her team: Electrical stimulation of the subcallosal cingulate region of the brain produced relief from treatment-resistant depression in not just 10 subjects with unipolar depression, but in seven with bipolar depression, when compared with a sham procedure in the same subjects. The findings were reported in the February Archives of General Psychiatry.
This most recent study, which was conducted at Emory University, where Mayberg is a professor of psychiatry, neurology, and radiology, included the following:
a screening phase of at least four weeks, | |||||
bilateral implantation of electrodes in subcallosal cingulate white matter and implantation in the infraclavicular region of a small device to deliver continuous electrical stimulation to the subcallosal cingulate, | |||||
a four-week phase during which subjects were told that they were being randomized to receive either active stimulation or no stimulation, but where none of them received stimulation, and | |||||
a chronic stimulation for up to two years. | |||||
After the four-week sham period, subjects’ depression scores showed a small (14 percent) but not clinically significant decrease compared with baseline values. In contrast, after 24 weeks, one year, and two years of chronic stimulation, the subjects’ depression scores were 44 percent, 43 percent, and 70 percent lower than at baseline, respectively.
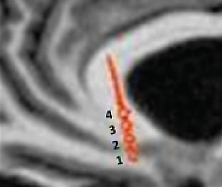
This postoperative CT scan shows where an electrode has been placed in the subcallosal cingulate region of the brain of a patient with refractory depression. The numbers label each of the four contacts on the electrode. Helen Mayberg, M.D., and her colleagues stimulate the contact that is best located in the subcallosal white matter.
After two years of chronic stimulation, response and remission rates were high (92 percent and 58 percent), and no patient was in a moderate or severe depressive episode. Furthermore, response rates were similar between bipolar and unipolar depression subjects. And there was no evidence that the treatment induced manic phases in the bipolar subjects.
“Taken together, these results support the long-term safety and antidepressant efficacy of subcallosal cingulate deep brain stimulation for treatment-resistant depression, building on previous reports of long-term efficacy in major depressive disorder, “ Mayberg and her team concluded in their report.
So far, 66 individuals with treatment-resistant depression have received this technique, Mayberg told Psychiatric News. In addition to the 37 whom she and her colleagues treated, 21 were treated in a pilot study conducted by St. Jude Medical Inc., which makes the stimulation device, and eight were treated in Spain. “The patients appreciate the opportunity to participate in this research,” she said. “They also appreciate getting their lives back, even though they realize that recovery is hard work after not being able to do anything for many years.”
St. Jude Medical Inc. is pursuing Food and Drug Administration (FDA) approval of its device for this indication, Mayberg said. “They are currently testing the safety and efficacy of subcallosal cingulate white matter deep brain stimulation for treatment-resistant depression in a multicenter, randomized, controlled … clinical trial.” It will be at least several years before such approval is obtained, she said.
“I think this is a very promising approach for some highly refractory patients,” Steven Dubovsky, M.D., chair of psychiatry at the University of Buffalo, told Psychiatric News. “I think it will be approved.”
Deep brain stimulation was approved by the FDA in 2003 to treat refractory Parkinson’s disease. In 2009 the FDA granted permission for it to be used to treat refractory obsessive-compulsive disorder as well, although in this case, the permission was granted under a humanitarian device exemption, not on the basis of clinical trials (Psychiatric News, March 18, 2011).
The study was funded by the Dana Foundation, the Stanley Medical Research Institute, the Woodruff Foundation, and Emory Healthcare. The deep brain stimulation devices were donated by Advanced Neuromodulation Systems/St. Jude Medical Neuromodulation. 
An abstract of “Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Unipolar and Bipolar Depression” is posted at http://archpsyc.ama-assn.org/cgi/content/abstract/69/2/150.



