FDA: Drug Studies Include Too Few Women, Minorities
Abstract
Though participation in phase 3 clinical trials for minorities has increased over the years, phase 1 and 2 trials—examining dosing and toxicity—remain less diverse, say experts in minority health issues.
Over the past two decades, some at the Food and Drug Administration (FDA) have raised questions about adequate inclusion of women and minorities in clinical trials that are part of the drug-approval process. The FDA has now responded to those issues.
Just before the end of 2014, the FDA released data from six clinical studies that included the percentage of participation by women and racial minority populations. “I am pleased by this action step by the FDA,” said Phyllis Greenberger, president and CEO of the Society for Women’s Health Research (SWHR). “The data validate the concern we have expressed to the FDA for 25 years.”
The newly released data are from clinical trials related to approvals of medications to treat a broad range of diseases—from bacterial skin infections to cardiovascular disease. The drugs were approved over a two-month period in 2014 as a part of the Drug Trials Snapshot, an FDA pilot project intended to provide information about the sex, age, race, and ethnicity of clinical participants for a small group of recently approved drugs. The creation of Snapshot was in response to the 2012 FDA Safety and Innovation Act, a law designed to encourage drug makers to provide safety and effectiveness data by demographic groups. The agency plans to publish a Snapshot for every new drug and original biologic that is approved, starting this year.
Greenberger told Psychiatric News that although the SWHR applauds the FDA for taking an initial step toward reducing health disparities and the lack of clinical trial participation from minority populations, the results from the pilot project are discouraging.
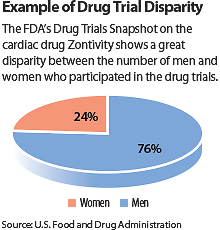
The data showed that Caucasians accounted for about 76 percent of participants for each clinical trial at baseline—while African Americans and Asian Americans, respectively, accounted for no more than 11 percent and 16 percent per trial. Half of the clinical trials reported that more than 85 percent of participants were Caucasian.
As it relates to gender, only one of the six clinical trials in the Snapshot project contained more female than male participants. Collectively, the remaining clinical trials had on average 27 percent more male participants than female participants at baseline.
“As demonstrated by the evidence, there is still work to be done with industry-sponsored trials,” Greenberger told Psychiatric News. She explained that although there has been more progress in recruiting women and minorities for phase 3 trials, there has been less progress in recruiting these groups for phase 1 and 2 trials, where dosing and toxicity are examined. “We know that dosages may need to be different among the sexes, which was clearly shown with Ambien and other similar drugs approved by the FDA,” she emphasized.
Greenberger also said that barriers—such as transportation issues, child care, and distrust of medical research due to unethical activities in the past—that keep women and racial minorities from participating in clinical trials need to be properly addressed in order to have a discussion on inclusivity and the redesign of the infrastructure of clinical trials.
With the FDA’s implementation of Snapshot, said Greenberger, “we think this is an opportunity to change the way clinical trials for drugs and devices are conducted. We hope that the information that the FDA posted showing that the results were not clinically relevant for a significant portion of the population will encourage, or hopefully compel, researchers to address demographic issues in future trials.”
The FDA is seeking feedback from the public on the content, format, and overall usability of Snapshot. ■
Information about the FDA’s Drug Trials Snapshot can be accessed here.