New TMS Device Reduces Depression Symptoms
Abstract
Synchronized transcranial magnetic stimulation, which delivers stimulation using lower energy than traditional repetitive TMS, may offer patients with depression a more comfortable alternative.
Findings from a study published in the summer issue of Brain Stimulation suggest that a new therapeutic option may be on the horizon for individuals with major depressive disorder (MDD). That therapy is synchronized transcranial magnetic stimulation (sTMS).
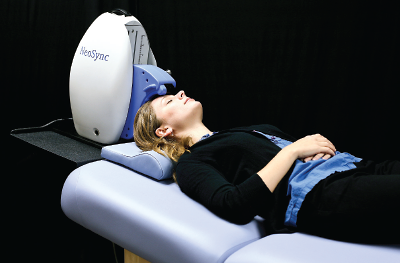
A new synchronized transcranial magnetic stimulation device called NEST by NeoSync is intended to treat symptoms of major depressive disorder by providing low-energy sinusoidal stimulation tailored to each patient’s brain activity. The device has not been approved by the Food and Drug Administration.
“Synchronized TMS is a new treatment technique for major depression that delivers transcranial magnetic stimulation using lower energy than traditional repetitive TMS [rTMS],” said the study’s lead author, Andrew Leuchter, M.D., a professor of psychiatry and director of the Neuromodulation Division at the David Geffen School of Medicine at the University of California, Los Angeles. The sTMS device, called NEST, contains three small rotating magnets that provide low-energy sinusoidal stimulation tailored to each patient’s brain activity.
“Before the first sTMS treatment,” explained Leuchter, “a brief, five-minute electroencephalogram recording is performed to measure the patient’s individual alpha frequency [IAF] in the brain. The sTMS device then rotates the magnets over the midline region [of the skull] at a frequency personalized to each individual. We believe that the IAF represents a ‘resonant frequency’ of the brain, and by delivering stimulation at each person’s unique resonant frequency, we can achieve therapeutic benefit using much lower energy [than rTMS] … which could be more comfortable for patients.”

Andrew Leuchter, M.D., believes that because synchronized transcranial magnetic stimulation (sTMS) has low energy emission, sTMS may potentially have fewer side effects than traditional TMS therapies.
To examine the efficacy, safety, and tolerability of NEST, Leuchter and colleagues performed a multisite, randomized study with 202 patients with MDD, in accordance with DSM-IV criteria, who were either treatment naïve or treatment resistant to pharmacotherapies for depression. Participants were required to be free of all medications known to significantly affect brain function for at least one week prior to enrollment to study endpoint. For a total of six weeks, patients underwent neuromodulation with an active NEST device or simulation of neuromodulation with an inactive NEST (placebo) device five days a week for 30 minutes. The primary outcome was a decrease in symptom severity, assessed by the 17-item Hamilton Rating Scale for Depression (HAM-D17).
Of the 120 people who completed the study, those receiving active device treatment had a significantly greater mean decrease in HAM-D17 scores—indicative of reduced symptom severity—than those receiving treatment from the inactive device. In addition, among patients in the active cohort, sTMS was observed to be twice as effective in reducing symptoms among participants who were unresponsive to previous drug treatment compared with those who were treatment naïve. The study noted that there were no device-related serious adverse events in either the active or inactive treatment groups; the primary reason for failure to complete the trial was difficulty in fulfilling visit requirement.
“The degree of improvement seen in subjects receiving sTMS is similar to that reported in previous treatment studies using rTMS or medications for MDD,” wrote the authors. However, “the results of this study cannot be formally compared to those of previous studies of rTMS or medication treatment because of the possible confounding effects of differences in study design. It therefore remains unknown whether individual patients may respond preferentially to one type of TMS device,” the researchers noted.
Though NEST has yet to be approved by the Food and Drug Administration for clinical use, Leuchter told Psychiatric News that neuromodulation technology is evolving rapidly, with many novel techniques on the horizon that may benefit patients with MDD. Because sTMS can be administered without significant risk and at a low rate of adverse events, Leuchter said that sTMS may constitute a valuable neuromodulation treatment for MDD.
The study was funded by NeoSync, developers of NEST. ■



