ADHD Treatment Arsenal Increasing Rapidly
 Novartis Pharmaceuticals won approval from the Food and Drug Administration last month to market its improved version of methylphenidate, the standard medication prescribed for attention deficit/hyperactivity disorder for nearly 50 years.
Novartis Pharmaceuticals won approval from the Food and Drug Administration last month to market its improved version of methylphenidate, the standard medication prescribed for attention deficit/hyperactivity disorder for nearly 50 years.
The new version, dexmethylphenidate, will be marketed under the trade name Focalin. Novartis expects Focalin to be available in pharmacies in late January.
Dexmethylphenidate is the latest drug to be approved in a string of medications either in the late stages of development or under current FDA review for the treatment of ADHD.
Dexmethylphenidate is the d- , or “right-handed,” isomer of the racemic mixture found in d,l-methylphenidate (Ritalin). It is one of several examples of medications designed through advancing technology aimed at isolating single active isomers (Original article: see story below). It was separated and developed for Novartis by Cellgene Corporation. Because dexmethylphenidate is the more active of the two isomers, doses are half that recommended for methyl-phenidate. The recommended starting dose for new patients is 2.5 mg, and patients being switched from methylphenidate should be started at half their final methylphenidate dose, according to Novartis’s research.
In company-sponsored clinical trials reviewed by the FDA, the drug was found to be effective in lowering scores on the Teacher SNAP-ADHD assessment rating scale. SNAP assessments by parents of children in the study supported the improvements indicated by teachers. In addition, patients receiving dexmethylphenidate significantly improved on Clinical Global Impression of Improvement scores. Interestingly, in the clinical trials there appeared to be a slight but statistically significant advantage in improvements reported with dexmethylphenidate compared with methylphenidate.
Overall, the side-effect profile appears to be the same as for methylphenidate, with the most commonly reported complaints being stomach upset and insomnia.
Like other stimulant medications used for ADHD, dexmethylphenidate is a Schedule II controlled substance.
Once-Daily Dosing
While dexmethylphenidate, like its parent compound, must still be prescribed in multiple daily doses, the pharmaceutical industry has been pursuing ways to allow patients with ADHD to take only one daily dose. For children, this would eliminate the stigmatizing need to take medication during the school day. In addition, sustained or controlled-release formulations are generally believed to give patients more consistent control of their symptoms throughout the day.
On November 5 Shire Pharmaceuticals launched its latest version of its mixed amphetamine salts product (Adderall). The new formulation, Adderall XR, is a new use of existing drug technology that encapsulates the active medication in coated beads within the capsule. In Adderall XR, half of the beads dissolve immediately, releasing a first dose of medication into the bloodstream, followed by the remaining half dissolving approximately four to six hours later. The formulation eliminates the need for patients to take a second, mid-day dose and allows for symptom control for eight to 10 hours.
The regular form of Adderall is the most prescribed ADHD medication, according to data from NDCHealth, an Atlanta-based independent firm that tracks drug sales. NDCHealth data for the 30 days ended November 1 showed Adderall with a 33 percent market share. Preliminary data from NDCHealth for the new formulation showed Adderall XR with a 5.4 percent market share by the end of its first four weeks on the market.
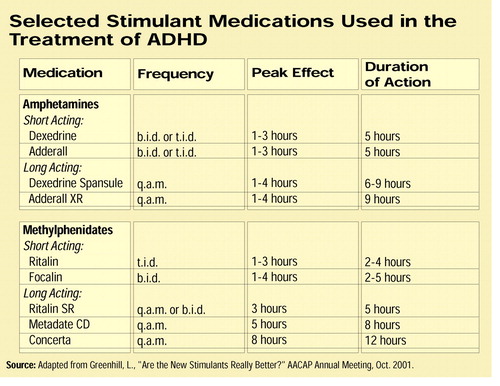
Adderall XR enters a market already strongly competitive with three other longer-acting methylphenidate modified-release products: Celltech Pharmaceutical’s Metadate CD; Concerta, marketed by Alza Pharmaceuticals; and Novartis’s Ritalin-SR. Each product uses slightly different patented technology to sustain the release of the active medication over the course of the day.
Comparing Options
A number of studies have compared the newer long-acting medications, according to Laurence Greenhill, M.D., a professor of clinical psychiatry at Columbia University and the New York State Psychiatric Institute. Greenhill reviewed the comparisons recently at the annual meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in Honolulu.
Greenhill emphasized that medication can be an effective adjunct in a comprehensive treatment plan for ADHD patients, which, he said, should include educational, behavioral, psychological, and family therapies, as well as parenting education.
In studies presented at the AACAP meeting and those reviewed by Greenhill, Metadate CD and Concerta were roughly comparable in terms of efficacy and safety of the two products; however, differences are apparent, Greenhill said.
Although the overall exposure to methylphenidate from the two products appears comparable, higher blood levels are achieved quicker with Metadate CD than with Concerta, he said. Blood levels of methylphenidate achieved with Metadate CD continued to be higher compared with Concerta through the six-hour time point in several published studies, according to Greenhill. However, at later time points, between eight and 12 hours, concentrations were higher with Concerta.
The seemingly minor difference in the amount of methylphenidate released with the two preparations over time does lead to a practical difference in how Greenhill uses the two medications.
“Concerta is the longest-acting preparation,” Greenhill told those attending his AACAP presentation, “and I use it in children who have no sleep problems on prior treatment [with stimulants and who have] a big problem with homework.”
The higher concentrations later in the day help patients to focus and complete homework tasks, but they can lead to insomnia. Nonetheless, “if I have a child who has a poor appetite, won’t eat dinner, and on past treatment has been sensitive to the anorexic effects [of stimulants], and is sleeping poorly, then I’d use something shorter like Metadate CD or Ritalin SR.”
Greenhill noted, however, that Ritalin SR has generally not been highly prescribed. Patients do not appear to achieve relief from symptoms as quickly with Ritalin-SR as they do with immediate-release methylphenidate and relief of symptoms throughout the patient population using Ritalin-SR has been shown to be not as consistent or as robust as compared with two doses of immediate-release methylphenidate. Although spokespersons for Novartis would not comment, it is widely believed by many clinicians that these studies were the impetus for Novartis’s development of its new Ritalin-LA (Original article: see box on page 17).
If the patient is experiencing dysphoria, a side effect of long-acting stimulant therapy, then Greenhill prefers to use Adderall XR, which he says does not seem to cause dysphoria.
Although Greenhill generally initiates stimulant therapy with an immediate-release preparation—to check for any allergic reactions or immediate adverse events—he strongly urges clinicians to move more patients to long-acting forms of medication therapy.
“There is enough strong evidence in the literature now to suggest they are effective,” he said. “It gets the medication out of the school and under the control of the parents.”
Several additional ADHD medications are either in the late stages of development or are pending FDA approval (Original article: see box on page 17).
“I’m looking forward to having important new options in the armamentarium. Increasing options for patients is always a good thing,” he said. ▪



