Brain Stimulation Promising In Resistant Depression
At APA's annual meeting in 2001 the buzz about new options for treatment-resistant depression primarily involved “new molecular targets” for pharmacotherapy—involving substances like glutamate, the neurokinin-1 receptor that binds substance-P, corticotrophin-releasing hormone, and neuropeptide-Y. Yet today, each of these “targets” has proven disappointing as treatment options for patients with treatment-resistant depression.
At APA's annual meeting this year, the buzz focused on brain stimulation devices and the promise they may—or may not—hold for those with difficult-to-treat depression.
“The need certainly remains great,” said psychiatrist Mark Demitrack, M.D., vice president and chief medical officer of Neuronetics Inc., a company that is developing a novel brain stimulation device. In May Demitrack chaired an annual meeting symposium—one of about a half dozen presentations on treatment resistance—titled “Difficult to Treat Depression: Looking for Our Keys Beyond the Light We Can See.”
Demitrack is overseeing the development of a novel technique using transcranial magnetic stimulation (TMS) as a potential treatment for resistant depression and possibly other psychiatric disorders.
Bridging a Wide Gap
There is little dispute that a significant proportion of patients with major depression do not respond adequately to current best-practice treatments. While the definition of “treatment resistance” can vary, the term is widely used to indicate a patient with depression who has not responded to at least two courses of antidepressant medications from two different classes. A treatment-resistant patient has also, by definition, taken each medication in adequate doses for an adequate period of time, as determined by FDA-approved labeling and practice guidelines.
Epidemiological data, Demitrack noted, indicate that of the more than 14 million persons in the United States with depression, only about half receive treatment. Of those who are treated, fewer than half obtain an adequate response.
“The real issue in treating resistant depression [is] that medications and electroconvulsive therapy.. .leave a significant gap [in both treatment options and effectiveness] that remains to be filled for these patients,” Demitrack told Psychiatric News in a follow-up interview.
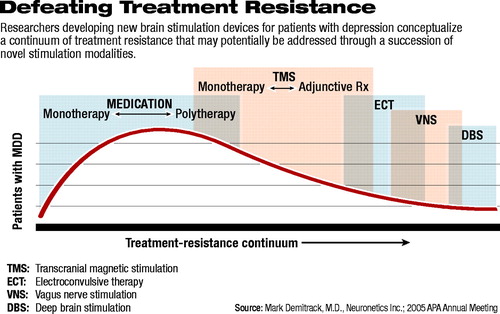
He described a “treatment-resistance continuum” (see diagram below) that is not adequately spanned by approved treatments. Medications, he explained, whether given as monotherapy or polytherapy, fail to elicit an adequate response for a significant proportion of patients and are associated with a wide array of adverse effects. Electroconvulsive therapy (ECT), although effective at relieving many patients' depression, often results in cognitive changes such as amnesia. In addition, some patients experience transient agitation or akathesia, which often resolves on its own or responds to medication.
Each of the brain stimulation modalities in development today, Demitrack said, is based on “the long-standing question of whether the benefits of electrical stimulation—as a method of treatment—could be separated from the actual physical convulsion.”
Demitrack continued, “We obviously have chemical methods of altering the brain's electrochemical signals. The remaining question has been, Can we provide electrical stimulation of sufficient intensity to cause neuronal depolarization and keep it within a specifically targeted, local area of the brain [in order to avoid seizure]? Now that we've shown we can do that technologically, the question is, Can we achieve a therapeutic benefit [on the patient's mood]?”
The modern form of TMS was developed in 1985 by British physician Anthony Barker, M.D., who developed the “focal magnetic coil.” Barker was the first to demonstrate in humans that it was possible to deliver a focused electrical stimulation using strong magnetic fields that resulted in functional activation of neuronal circuits in the cortex.
Numerous researchers have since confirmed the technique's ability to produce localized stimulation, specifically of the brain's major mood circuits—primarily targeted in the prefrontal cortex.
Research has also documented that the same type of stimulation can produce limb movements when focused on the motor cortex and visual disturbances when focused on the visual cortex.
The advantages of TMS include its non-invasive nature. To date, few if any adverse effects have been associated with the treatment. Case reports from patients undergoing MRI, which uses similar strong magnetic fields focused on the body's anatomical structures, have indicated an associated antidepressant effect, providing further support for the efficacy of the potential treatment modality.
While TMS appears to be promising, it is still experimental and at best a year and a half away from approved use in the clinical setting. Early clinical trials have provided encouraging results, and Demitrack's company is recruiting subjects for a large, multisite clinical trial. The company aims to enroll 300 patients at 25 sites worldwide.
Neuronetics hopes to have results from its clinical trials early next year, Demitrack told Psychiatric News. “We hope to have an application for approval submitted to the FDA and have TMS on the market as soon as late 2006.”
Alternatives More Invasive
The other brain stimulation modalities in development involve more invasive technology and potentially more adverse events.
Vagus nerve stimulation (VNS), being developed by Cyberonics Inc., is the closest to marketing approval in the United States; it is already approved for use in European Union countries as an adjunctive treatment for depression in patients who have failed to respond to at least four adequate courses of antidepressant therapy.
VNS involves the implantation of a device similar to a cardiac pacemaker in a pocket cut into the patient's pectoral muscle. The device is attached to electrodes that are threaded under the skin and up to the neck, where they are attached to the vagus nerve. The device delivers programmed low-intensity electrical impulses to the nerve 24 hours a day. Clinical-trials data indicate that over the long term VNS reduces depression symptoms when used in conjunction with pharmacotherapy.
However, not all patients benefit from VNS, and few seem to achieve significant acute benefits—a finding that appeared to be a stumbling block with FDA regulators reviewing Cyberonics' application for approval. The company did receive an approvable letter for the device in February 2004 and expects final approval this summer (Psychiatric News, June 17). Cyberonics declined to comment for this article after issuing a press release indicating that it had “entered a formal quiet period with regard to all public communications pending notification by FDA of its final decision.”
Because VNS-device implantation requires a surgical procedure, complications such as wound infection and bleeding can occur. Patients also have experienced adverse effects from stimulation of the vagus nerve, nearly as wide-ranging in scope as the nerve's functions in the body. Nausea, numbness, tingling, and dizziness have been reported.
Deep brain stimulation (DBS) is similar in concept to VNS but involves implantation of electrodes directly into the brain. Risks of DBS surgery include intracranial bleeding and stroke, infection, and loss of function.
In the United States DBS is approved only for the treatment of Parkinson's disease, while VNS is approved only for the treatment of epilepsy.
Each of these treatment modalities, Demitrack noted, has helped researchers further understand the basics underlying the anatomy and physiology of depression.
Foremost, Demitrack said, “We've moved away from looking at depression in the acute infection paradigm and finally realized that it is more diabetes-like: it is a chronic, remitting/relapsing disease.”
But the real challenge, Demitrack acknowledged, “is to have the clinical-development activities actually catch up with the advancing theoretical development.”
With new technologies, he concluded, “we are forced to bring some very different threads of evidence [regarding the basis of depression] together and think differently about what it will take to solve the very significant treatment gap we currently have.” ▪



