FDA Catching Up With Drug Companies That Push Online Envelope
Fourteen pharmaceutical companies have received warning letters from the Food and Drug Administration (FDA) about violating direct-to-consumer (DTC) advertisement regulations in their paid links on Internet search engines.
In recent years the pharmaceutical and medical-device industries have been aggressively expanding marketing campaigns into online media. The FDA has had to step up its monitoring and regulatory actions in response (Psychiatric News, March 20).
Sent on April 2, the most recent batch of warning letters from the agency's Division of Drug Marketing, Advertising, and Communications targeted a number of major pharmaceutical companies over dozens of prescription drugs they were advertising online. The agency's objections focused on sponsored links and accompanying short statements posted on Google that were intended to attract Internet users to visit promotional Web sites for the products (see screenshot).
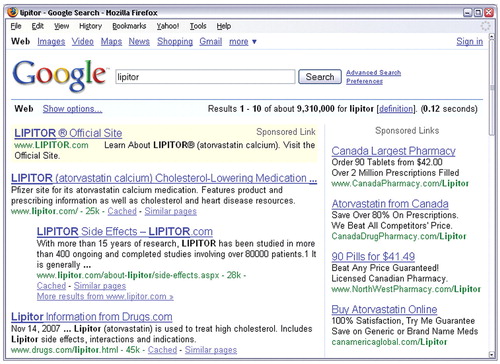
Several drugs named in these warning letters are often prescribed in psychiatry, including Forest Laboratories' acamprosate, escitalopram, and memantine; Pfizer's pregabalin; Boehringer Ingelheim's pramipaxole; and Eli Lilly's duloxetine.
Eli Lilly, for example, was criticized for suggesting the efficacy of duloxetine in the online blurb “Learn about an SNRI for depression called Cymbalta® (duloxetine HCl),” along with a link to the product's Web site, without giving any information about potential adverse events and safety risks. Forest Laboratories' links for several drugs, including memantine, donepezil, and escitalopram, “inadequately communicate[d] the drugs' indications ... and fail[ed] to use the required” generic names.
The other warning letters cited similar problems with not including adequate information about safety risks and complete indications.
The use of “sponsored links” that are posted on the pages of Internet search engines has become a popular marketing strategy for companies and organizations to promote their Web sites and products. It is inexpensive and has the advantage of targeting advertisements to people who are already looking for information about a particular product or related subjects such as disease information.
However, these sponsored links are given limited space (95 characters by Google) for a statement to accompany the link. The FDA's warning letters have made it clear that the agency is sticking to its rules for all public-access pharmaceutical promotional materials and requires a complete and balanced representation of both efficacy and safety information when a specific product is advertised. Web sites' length limit for sponsored links is likely to make it impossible for companies to go much beyond stating drug names.
As have other companies in other industries, pharmaceutical and medical-device manufacturers have been testing various ways to expand their online marketing presence. In addition to YouTube video postings and channels, companies are making more use of online social networks such as Facebook and MySpace to connect with consumers directly. For example, McNeil Pediatrics, a division of Johnson & Johnson, has launched Facebook pages, or“ online communities,” for adults with attention-deficit/hyperactivity disorder (ADHD) and for mothers of children with ADHD. McNeil's ADHD drug methylphenidate hydrochloride extended-release tablets (Concerta) won FDA approval for treating adults in June 2008.
The FDA warning letters to pharmaceutical companies are posted at<www.fda.gov/cder/warn/warn2009.htm>.▪



