What Is the Link Between SSRIs and Fear Extinction?
Abstract
A new preclinical study opens doors to better understanding of the role of antidepressants in extinction of learned fear.
Antidepressant treatment impairs fear extinction in rats, a finding that may—or may not—point the way to new insights into fear-based psychiatric conditions such as posttraumatic stress disorder (PTSD).
“Extinction does not destroy original fear memories but instead involves learning new information about the relationship between the [conditioned stimulus] and the [unconditioned stimulus],” wrote Nesha Burghardt, Ph.D., a postdoctoral researcher at Columbia University College of Physicians and Surgeons, and colleagues online December 20, 2012, in Biological Psychiatry.
The best-tested model of extinction in humans is exposure therapy, which is often accompanied by other treatments, typically an SSRI.
“Long-term SSRI treatment is known to be effective in the treatment of anxiety disorders,” Burghardt told Psychiatric News. “So I expected chronic citalopram treatment would decrease expression of fear in rats. I was surprised to find that there was no effect on fear expression and instead an impairment in extinction learning.”
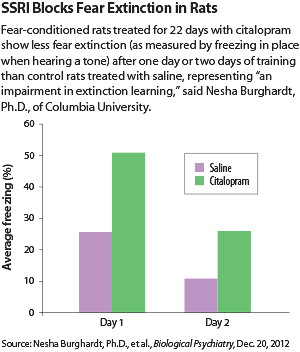
Burghardt and colleagues treated fear-conditioned rats with a course of the SSRI citalopram, the antidepressant tianeptine, or saline for either 22 or nine consecutive days. Tianeptine is a selective serotonin reuptake enhancer that works through the glutamatergic system. It is approved in Europe but not in the United States.
Administering either drug chronically (22 days) impaired the acquisition of fear extinction, but subchronic use (nine days) did not. Apparently, rats, like many humans, take a while to display the effects of antidepressants.
“In this way, our rat studies parallel clinical work and provide insight into some of the molecular changes that occur with long-term treatment in people,” Burghardt said.
The study may have used rats as subjects, but it raises fundamental questions for the clinical field, said K. Luan Phan, M.D., a professor of psychiatry and director of the Mood and Anxiety Disorders Program at the University of Illinois at Chicago. Phan was not involved in the fear-extinction study.
“If the patient takes a medication while undergoing exposure therapy, does it help or hinder in the long run?” Phan asked. “These animal studies will in part help us understand the mechanisms of fear extinction as the results of clinical trials are published.”
More clinical trials may be needed to test whether patients with PTSD receiving prolonged exposure therapy should get SSRIs at the same time.
Phan, with other researchers, is now in the early stages of testing that question in a clinical trial. The four arms of the trial involve comparisons of an SSRI vs. exposure therapy vs. a combination of the SSRI and exposure vs. exposure plus placebo.
Combining SSRIs and cognitive-behavioral therapy is already known to have an additive and possibly synergistic effect in major depressive disorder, he said. For anxiety disorders, adding a drug is sometimes equal, sometimes better, sometimes worse.
Results have been mixed in fear extinction, even in rodents. A 2011 study in mice found that antidepressant drugs helped with fear extinction, while the current study in rats finds just the opposite.
“We do know that benzodiazepines should not be used if a patient is going on exposure-based therapy,” said Phan.
The impairment of extinction learning induced by citalopram was also associated with decreased expression of the NR2B subunit of the N-methyl-D-aspartate [NMDA] receptor in the basal and lateral nuclei of the amygdala, said the researchers.
“Our data suggest that the antianxiety and antidepressant effects of SSRIs may depend for their effects on the NR2B subunit of the NMDA receptor,” said Burghardt. This mechanism may also explain the drugs’ potential to impair extinction learning.
“Based on our results, it would be ideal to develop a drug that selectively activates the NR2B subunit of the NMDA receptor in the amygdala, to facilitate extinction learning,” she said. D-cycloserine is an NMDA partial agonist that has proved therapeutically effective as an adjunct to exposure-based therapy, suggesting that focusing on the glutamatergic system to facilitate fear extinction is a promising avenue of research.
Funding for the study was provided by the National Institutes of Health. ■
An abstract of “Chronic Antidepressant Treatment Impairs the Acquisition of Fear Extinction” is posted at http://www.biologicalpsychiatryjournal.com/article/S0006-3223(12)00925-0/abstract.



