Folate Gene Variants May Influence Negative Schizophrenia Symptoms
Abstract
Schizophrenia’s negative symptoms may be due, at least in part, to low-functioning folate gene variants. Folate supplements might help counter such symptoms.
Two B vitamins—choline and folate—may play critical roles in schizophrenia, growing scientific evidence suggests.
Choline supplementation during pregnancy facilitated neonatal development of cerebral inhibition—a critical function related to sensory gating and attention. Since individuals with schizophrenia have defects in sensory gating and attention, choline supplementation during pregnancy or perhaps early in life might be able to counter the development of such defects (Psychiatric News, February 1).
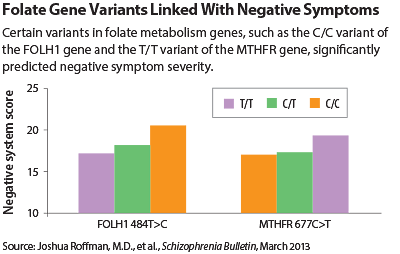
Now multiple gene variants within the folate metabolism pathway have been linked with the negative symptoms of schizophrenia, suggesting that folate supplementation might be able to counter such symptoms.
The lead scientist of the new study was Joshua Roffman, M.D., an assistant professor of psychiatry at Harvard Medical School. The findings appeared in the March Schizophrenia Bulletin.
Reduced maternal folate intake during pregnancy has been linked with an increase in schizophrenia risk. Low blood levels of folate have been observed in schizophrenia subjects and have also been linked with negative symptoms. Moreover, variants in two genes that code for enzymes that metabolize folate have been linked with schizophrenia risk. Thus Roffman and his colleagues wondered whether any of the specific variants of genes that code for enzymes that metabolize folate might be linked with negative symptoms.
They rated 82 subjects with schizophrenia on negative-symptom severity. They then genotyped the subjects for six common variants across five genes known to regulate folate metabolism as well as for a variant of the COMT gene known to interact with one of the folate gene variants. They then assessed whether any of the seven gene variants could be significantly linked with negative-symptom severity.
Four variants could. They were variants of the MTHFR, MTR, FOLH1, and COMT genes. Moreover, all four of the variants were low-functioning ones, indicating that they weren’t doing an adequate job regarding folate metabolism.
“However, the net effects of these low-functioning variants on negative symptoms were somewhat mitigated among patients who had higher folate levels in their blood (which largely reflects dietary intake),” Roffman told Psychiatric News. “This pattern suggests that increased dietary folate may rescue some patients who are genetically predisposed to negative symptoms due to abnormal folate metabolism.”
This idea was supported by a pilot study of folate supplementation that Roffman and his colleagues conducted, in which schizophrenia subjects who carried one of the low-functioning folate gene variants—a variant of the MTHFR gene—were more likely to show improvements in negative symptoms than were schizophrenia subjects who carried the normal version of the gene.
To find out whether increased dietary folate could actually help some patients who are genetically predisposed to negative symptoms due to abnormal folate metabolism, it was necessary to conduct a larger multisite, randomized, placebo-controlled trial. The cohort Roffman and colleagues studied in this research included 140 subjects with schizophrenia. Half received folate plus vitamin B12 supplements, and half received a placebo for 16 weeks. The subjects were tested for negative symptoms at the start and at the end of the trial.
However, their results—reported March 6 in JAMA Psychiatry—were not clear cut.
Although the negative symptoms of the subjects getting the folate and vitamin B12 supplements did improve somewhat over the trial period, the improvement was not significantly better than that experienced by the placebo group. However, subjects who got the supplements and who had two copies of a variant of the FOLH1 gene did show significant improvement in negative symptoms compared with the placebo group. Yet it was the high-functioning FOLH1 gene variant—not the low-functioning one—that had been previously linked with negative symptoms.
Roffman believes that more research needs to be done on this provocative but complex subject.
The initial research conducted by Roffman and his colleagues was funded by the National Institute of Mental Health, the Howard Hughes Medical Institute, the Charles King Trust, the Hall/Alden Trust, and the Harvard-Massachusetts Institute of Technology Division of Health Research and Technology.
The multisite, placebo-controlled study was funded by the National Institutes of Health, the Howard Hughes Medical Institute, and Harvard University. ■
An abstract of “Genetic Variation Throughout the Folate Metabolic Pathway Influences Negative Symptom Severity in Schizophrenia” is posted at http://schizophreniabulletin.oxfordjournals.org/content/39/2/330.abstract.
An abstract of “Randomized Multicenter Investigation of Folate Plus Vitamin B12 Supplementation in Schizophrenia” is posted at http://archpsyc.jamanetwork.com/article.aspx?articleid=1660588.



