Researchers Move Closer to Targeted Drug Delivery to Brain
Abstract
A new remote-controlled device being tested in animals offers researchers the chance to study the ways pharmacological and optical manipulations alter behavior.
One of the drawbacks of a standard psychiatric medication is a lack of selectivity; once a pill is ingested, one is at the mercy of human physiology to hope that enough of that agent reaches the desired brain region without too many unwanted interactions.
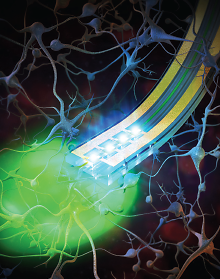
The wireless neural device developed by researchers at Washington University and University of Illinois features a dual-purpose probe that can stimulate through chemicals and light.
Targeted drug delivery to the brain is challenging, as very few biological compounds can cross the blood-brain barrier. While surgical implants that reach a specific part of the brain are an option, such technology can be bulky.
However, researchers from Washington University School of Medicine and the University of Illinois at Urbana-Champaign now say they have developed a tiny wireless device capable of delivering drugs to the brain with the push of a remote control.
While the device is currently only outfitted for mice and rats, the group says it represents the first wireless fluid delivery device for deep brain stimulation.
The delivery system is a lightweight plastic case (only 1.8 grams) containing four fluid chambers that can be attached to the skull of the animal like a cap with an ultrathin probe extending into the brain region of interest. When activated by remote, tiny pistons push the fluid down through the probe (The probe is thinner than a human hair to reduce potential damage in the brain).
Researchers have been using implanted devices for drug delivery in lab animals for a while now, but these systems require the animals to be tethered to pumps and tubes. For Michael Bruchas, Ph.D., an associate professor of anesthesiology and neurobiology at Washington University and co-leader of this study, such conditions were problematic for his research.
“My interests lie in how stress can be both a motivating property but also exacerbate or even cause psychiatric disorders,” he told Psychiatric News. In an effort to minimize the amount of extraneous stress the animals were exposed to, Bruchas teamed up with John Rogers, Ph.D., a professor of materials science and engineering at the University of Illinois at Urbana-Champaign to develop a wireless device that would allow animals to roam freely.
In addition to transporting fluids, the ultrathin probes developed by the team are outfitted with tiny LED lights, which allow the researchers to make use of the emerging technology of optogenetics, whereby neurons can be stimulated by flashes of light.
“It’s been difficult to merge these two techniques—pharmacological and optical manipulation—in research studies, but with this device we can integrate them,” Bruchas said.
As a proof of integration, the researchers used light to trigger the release of dopamine in the nucleus accumbens of mice, which stimulated the animals’ reward-seeking behavior. With a second press of a button, they released a dopamine blocker from the chambers to the same region and inhibited the light-induced reward behaviors.
“I am biased, but I do think the system works great and has real clinical potential,” said co-first author Jordan McCall, Ph.D., M.P.H., a former neuroscience graduate student in Bruchas’ lab who led the way on this project along with the University of Illinois’ Jae-Woong Jeong, Ph.D., a postdoctoral fellow at the time and now an assistant professor at the University of Colorado, Boulder.
“Still, there are many improvements needed for this technology to scale beyond rodents,” McCall continued. “Greater access to fluid would be a top priority, as right now we have only four reservoirs, so basically only four doses of medicine.” McCall noted that efforts are under way to modify the chambers to make them replaceable, like inkjet printer cartridges.
Battery life is another area of improvement, especially if these devices are to be implanted under the skin to make them less visible.
One of the beauties of the device design, Bruchas noted however, is that much of it was built from parts that can be found in hobby stores or designed using 3-D printing applications. “So almost any academic lab that might be interested in this technique could build their own and start tweaking it to make improvements,” he said.
The full details regarding the construction of the remote-controlled device were published July 16 in the journal Cell.
This study was supported by multiple grants from the National Institutes of Health, as well as from a National Security Science and Engineering Faculty Fellowship of Energy and an award from the U.S. Department of Energy Division of Material Sciences. ■
An abstract of “Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics” can be accessed here.



