Experts Debate What's Next for DBS for Depression
Abstract
A pair of pivotal clinical trials evaluating deep brain stimulation for patients with treatment-resistant depression found those who received stimulation were no better off than those who did not. Why not? That depends on whom you ask.
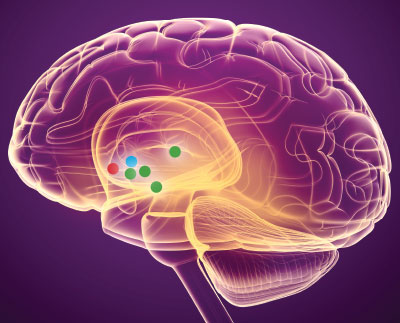
Though the subcallosal cingulate gyrus (red) and ventral capsule/ventral striatum (blue) were the target of the two largest DBS clinical studies for depression, several other brain regions have been tested as key targets for brain stimulation (green). There are no biomarkers to predict which region (if any) would be optimal for individual patients.
All surgeries—from a tonsillectomy to a coronary bypass surgery—come with risks. Deep brain stimulation (DBS), which involves surgically implanting 1 mm electrodes into the brain to stimulate nearby neurons, is no different.
The approach is approved for neurological problems associated with Parkinson’s disease or essential tremor. Some chance mood observations in patients with severe obsessive-compulsive disorder who received experimental DBS 20 years ago spurred a belief that DBS could be a much-needed breakthrough for patients with chronic depression that was resistant to conventional treatments.
After some encouraging findings emerged from preliminary (and not rigorously controlled) studies, researchers undertook a pair of large, placebo-controlled trials of DBS in patients with treatment-resistant depression starting in 2008. Both trials—known as BROADEN and RECLAIM—were stopped early after the data revealed patients who received active stimulation were no better off than those who did not receive active stimulation. Yet many medical professionals—including some involved in the failed studies—still believe that surgically stimulating brain tissue has a clinical future.
Given the current knowledge about the procedure, experts are asking themselves: Do the benefits outweigh the risks?
In the second half of a two-part series, Psychiatric News explores why the BROADEN and RECLAIM studies may have failed to demonstrate the benefit of DBS for patients with treatment-resistant depression and what the future may hold for this procedure.
Follow-up Trials Needed
“Is DBS ineffective for depression? I don’t know the answer to that,” said Donald Malone, M.D., chair of psychiatry at the Cleveland Clinic and a lead investigator on the RECLAIM study. “It’s a question that I think is worth exploring more.”
Malone acknowledged that his trial did not demonstrate a difference between active DBS and the sham procedure, but he said this might have been because the sham exerted a strong placebo effect. (The patients assigned to the sham DBS group underwent DBS surgery, but their electrodes were not turned on until after the study ended.) Since exploring DBS for depression was also a new paradigm at the time, it’s possible that the patient selection criteria were not right and/or the studies did not track changes in depressive symptoms long enough after patients received the stimulation, he added.
Malone suggested a longer study may be needed to evaluate whether DBS works. RECLAIM lasted 16 weeks, which is considered a sufficient amount of time for evaluating an antidepressant in a typical patient with depression. But it might take a year or more for someone who has been chronically depressed for potentially decades to show a response (BROADEN lasted for 24 weeks). Nonetheless, “it’s hard to justify performing surgery on someone and then withholding the treatment for a year,” he said, referring to the sham treatment process.
Another option for testing the effectiveness of DBS is to do a discontinuation study—every participant gets active DBS, and then at some point half of the participants have their devices turned off. This approach removes the potential placebo effects of the surgical procedure, but there are risks. Studies of DBS in Parkinson’s patients suggest that if the electrodes are shut off after an extended period, depressive symptoms can rapidly emerge.
In addition to the risks of these long-term studies, such studies would come at enormous costs, Malone noted. “These procedures are time, labor, and resource intensive, and I don’t know if companies are eager to sponsor another large trial,” Malone continued. “Our research suggests that about 40% of patients responded to DBS and are still doing well years later, and in psychiatry that’s a good number.” Still, without data to support the technique being better than sham, he thinks the research needs to proceed with great caution.
Helen Mayberg, M.D., director of The Nash Family Center for Advanced Circuit Therapeutics at the Icahn School of Medicine at Mount Sinai and lead investigator on the BROADEN study, remains more optimistic about DBS for the treatment of depression, but she agrees that DBS patients may need more time in treatment before they experience positive changes.
“These patients have been ill for so long that they need behavioral rehabilitation in addition to the stimulation,” she said. She and her team have continued to follow many patients who participated in her clinical studies and are continuing to receive DBS.
“We have seen about 50% of our patients respond after two years, and about 70% after four years,” she said. “If you got better, you stayed better.” These long-term data were published last October in the American Journal of Psychiatry.
Long-term Data Show Promise, Pitfalls
“I have to give a lot of credit to Mayberg and her colleagues for reporting these long-term results,” said Stanley Caroff, M.D., an emeritus professor of psychiatry at the University of Pennsylvania Perelman School of Medicine. He noted that once clinical trials are completed, the long-term outcomes of participants are rarely reported, so providing well-documented follow-up for eight years is informative.
Still, Caroff questions some of the conclusions offered by Mayberg and colleagues in the paper that “most participants experienced a robust and sustained response.” He noted that most of the participants still fluctuated in depression severity over time, with only 21% reporting continuous improvement of 50% or greater since their first year of participation in the trial. They were also receiving other medications or psychotherapy that may have helped prevent depression recurrence, and with no controls for comparison, the exact contribution of the brain stimulation to the effect is unknown. Caroff said that he also felt the article downplayed some of the adverse outcomes of DBS, which included surgical side effects such as infections, as well as device malfunctions, psychiatric hospitalizations, and suicide attempts.
Robert Coffey, M.D., a retired neurosurgeon who worked at Medtronic when the company conducted the RECLAIM trial, added that these new data and other long-term reports of DBS are hindered by a fatal flaw: The studies only assess the patients who are still coming to the clinic. “What has happened to all the people who are no longer showing up after eight years?”
Caroff also highlighted that there is no consensus on which brain regions are optimal for stimulation. BROADEN and RECLAIM each targeted a different location, and several other areas have been considered or tested in pilot studies. Currently, there are no biomarkers to suggest which region might be the best choice for individual patients.
“Given the safety concerns, the evidence is insufficient to recommend DBS as a depression treatment, in light of the many other approved options available,” Caroff said. He pointed to the recent approval of ketamine and brexanolone for treatment-resistant depression and treatment-resistant postpartum depression, respectively, as examples of clinical progress in the field.
Personalizing DBS in the Future
Malone emphasized that depressed individuals who are DBS candidates are in a different class than those who would benefit from medication, psychotherapy, or even noninvasive stimulation therapies such as transcranial magnetic stimulation.
“Some of the participants in our study had tried and failed 12 different treatments, including ECT [electroconvulsive therapy], often seen as a last-resort treatment,” he said. “These patients have a condition so pervasive that it is disabling.”
DBS, Mayberg said, offers a potential solution for patients with few other options. “I compare people with depression to those with arrythmia,” Mayberg added. “Sometimes medications are adequate to restore the heart’s electrical circuitry, but some people need surgery to implant a pacemaker. DBS is a similar strategy for the brain’s circuits.”
Similar to the way that Thorazine was used in the 1950s to help stabilize patients in spite of the medication’s side effects, Mayberg said she believes DBS can help stabilize patients with treatment-resistant depression so they can respond better to other therapies.
Given the continual advances in brain imaging and computational biology, Mayberg said she is also hopeful that researchers will become better at personalizing DBS and finding the best electrode placement for each patient. Newer implants could also offer longer battery life and internal sensors that can adjust the voltage automatically depending on brain activity. (Currently patients come in for visits every few months to have stimulation level manually adjusted.)
“I believe there are exciting advances on the horizon,” she said. Some in the psychiatry field might disagree, but it will be interesting to see how this story plays out. ■
“A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression” is posted here.
“Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression: A Multisite, Randomised, Sham-Controlled Trial” is posted here.
“Long-Term Outcomes of Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression” is posted here.



