Pluripotent Stem Cells May Unlock How Genetic Variants Result in Mental Disorders
Abstract
Stem cell–derived brain cells are helping psychiatric researchers understand how genes influence neurodevelopment and thereby impact risk for psychiatric disease.

Scientists in a variety of disciplines have been exploring the biological basis of mental illness for more than a century. However, meaningful progress has been hampered by the complexity of the brain and the limited accessibility of live human brain tissue for experimental study.
In medical disciplines such as dermatology, infectious disease, and oncology, doctors take biopsies or other tissue samples to study what has gone wrong to cause disease. Scientists working in these fields can even culture disease-relevant cells to better understand the molecular pathways driving various disorders.
For obvious reasons, psychiatrists do not take brain biopsies from patients. Advances in brain imaging techniques have partially filled the gap, but brain imaging is still limited with respect to its resolution and to the specificity of functional activities it can depict. Postmortem brain studies, while informative, also have limitations: They do not contain living, functional brain tissue, which precludes experiments on whether or not a particular manipulation leads to a predicted response.
This highlights the fundamental problem that has hindered research on psychiatric illness: There is only so much that scientists can do to study the brain without performing experiments on the people to whom the brains belong. Without a doubt, much has been learned about the mind by studying how the brain responds to different psychological and chemical stimuli, but many types of experimental approaches—such as drug screens and genetic manipulations—would require gross ethical violations if they were performed on the brains of living people.
This is why the generation of human brain tissue through human-induced pluripotent stem cells (hiPSCs) is a potential game changer. Scientists can now create human brain tissue from readily accessible cells from skin and blood. These stem cell–derived brain cells can include relatively homogenous populations of neural cells or more physiologically relevant three-dimensional “mini-brains” termed organoids that allow investigators to study more complex aspects of neurodevelopment and circuits. These techniques enable scientists to study mental illness in new ways, focusing on how genes influence neurodevelopment and thereby impact risk for psychiatric disease.
hiPSCs Link Genetic Variants to Disease Pathways
The Psychiatric Genomics Consortium has identified hundreds of genetic variants that are associated with either an increase or decrease in risk for psychiatric disorders. Importantly, these variants appear to be overrepresented in pathways previously connected to brain disorders, including brain development, synapse-formation, neurotransmitters, learning and memory, and the immune system.
However, relying on genomics alone has important limitations for the following reasons:
Psychiatric disorders are highly heritable, and this genetic risk is “polygenic,” reflecting inherited risk impacting multiple genes.
With the exception of rare cases, the genetic variants associated with disease risk have small effect sizes individually, making it incredibly difficult to identify traditional “candidate genes” for mental illnesses.
There is significant overlap in the polygenic risk for psychiatric conditions. For instance, schizophrenia and bipolar disorder share many common risk variants.
Finally, psychiatric risk variants are highly overrepresented in parts of the genome that do not code for proteins, making the identification of the affected molecular pathways even more challenging.
The question remains: Exactly how do genetic variants lead to psychiatric disease? This is where in vitro models can be transformative. Increasingly, patient-specific hiPSC-derived brain cells are providing a new avenue to link specific variants to the genes and molecular pathways they affect.
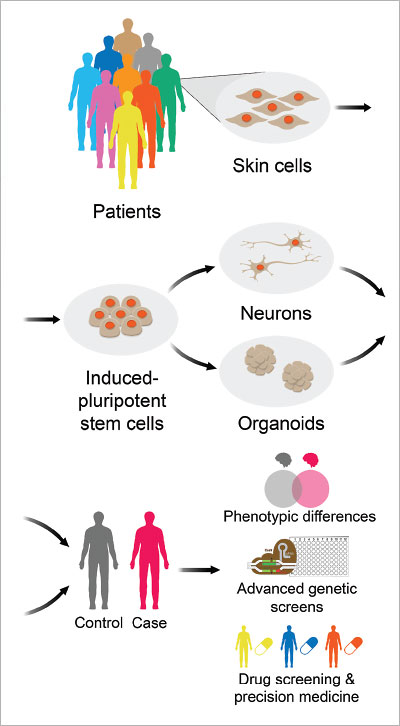
Using human-induced pluripotent stem cells (hiPSCs), scientists can create homogenous populations of neural cells, or even three-dimensional “mini-brains” termed organoids, that allow investigators to study more complex aspects of neurodevelopment and circuits.
Much of this work involves the use of CRISPR-based genome engineering. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) enables scientists to precisely edit the DNA of any genome.
Studies of rare, highly penetrant variants implicated in small numbers of psychiatric patients are revealing key insights into disease biology. In some cases, investigators studied hiPSC-derived brain cells from patients harboring rare variants and from unaffected controls; in others, genetic engineering approaches using CRISPR have been used to introduce a particular variant into healthy donor lines and compare neurons made from these edited and unedited hiPSCs.
In one pioneering example published in Nature in August 2014, Zhexing Wen, Ph.D., and colleagues at Johns Hopkins engineered hiPSCs to contain a mutant copy of the Disrupted in Schizophrenia 1 (DISC1) gene that was identified in a family containing high rates of mental illness. Upon differentiation of the hiPSC into neurons, investigators observed several abnormalities in synaptic functioning, thus linking a specific disease-associated mutation to relevant dysfunction in neural tissue.
An alternative method is to use CRISPR to edit different versions of a risk variant into the same donor line, then compare otherwise identical (or “isogenic”) neurons according to their risk genotype. In an article published in Nature Genetics (September 2019), Nadine Schrode, Ph.D., one of the authors of this article, Kristen Brennand, Ph.D., and colleagues applied CRISPR techniques to edit a single base pair variant predicted to have a causal role in schizophrenia. They evaluated neurons carrying either the wild type (“reference”) and edited (risk) variant, to control for differences in genetic background between individuals. They found that conversion of a noncoding single nucleotide polymorphism (SNP) resulted in significant changes in gene expression, neuronal morphology, and neuronal activity.
In the same study, the investigators also simultaneously manipulated small numbers of risk genes and observed synergistic effects that suggest important interactions between risk variants above and beyond the simple additive effects alone. Overall, this work highlights the promise of hiPSC models to pinpoint not just the cell-type–specific impact of single risk variants but also the interactions between them.
Many common variants associated with psychiatric disorder risk are believed to regulate gene expression, but it remains unclear which genes are targeted by each variant and the mechanisms by which each variant alters gene expression. By integrating CRISPR techniques with hiPSC-based models, mechanistic hypotheses can be evaluated in order to causally link genetic variants to changes in gene expression and neuronal function
Can hiPSCs Advance Precision Medicine in Psychiatry?
The ultimate allure of hiPSC-based models of psychiatric disease is the promise of improved treatments and tailoring them to individual patients. In the first proof-of-concept report of hiPSC neurons generated from patients with schizophrenia published in Nature (April 2011), Brennand, Fred Gage, Ph.D., and colleagues were able to partially improve synaptic deficits observed in patient-derived neurons with the antipsychotic loxapine.
Could hiPSC models be the breakthrough that finally enables biologically informed treatment discovery?
As an example, in an article in Nature Communications (October 2018), Brennand, Benjamin Readhead, Ph.D., and colleagues performed a drug screen on hiPSC-derived neural cells from schizophrenia patients and control patients and on eight unrelated cancer cell lines. Across these various cell types and donor lines, several thousand “transcriptional signatures” corresponding to drug response were derived. Using these gene expression–based readouts, the investigators found several drugs that reversed transcriptional abnormalities previously observed in postmortem studies of schizophrenia.
In the future, similar approaches may be used to link genetic information to predictions of differential treatment response, enabling clinicians to precisely match treatments to individual patients.
Use of patient-specific brain cells from hiPSCs is a transformative innovation that may one day yield new therapies for patients with psychiatric disorders. By studying patient-specific neurodevelopment and activity with hiPSC-based models, scientists are uncovering how genetic factors impact neuronal function, thus revealing key mechanisms of psychiatric disease. Our hope is to one day tailor psychiatric medicine to biologically informed treatments and bring relief to those suffering from mental illness. ■
“Synaptic Dysregulation in a Human Ips Cell Model of Mental Disorders” is posted here.
“Synergistic Effects of Common Schizophrenia Variants” is posted here.
“Modeling Schizophrenia Using Human Induced Pluripotent Stem Cells” is posted at here.
“Expression-Based Drug Screening of Neural Progenitor Cells From Individuals With Schizophrenia” is posted here.



