Guidance on Navigating Insurance Plans for TMS-Eligible Patients

When should transcranial magnetic stimulation (TMS) be considered for patients with depression? Which patients are covered by insurance for TMS?
The answer to the first question is fairly straightforward; however, the answer to the second question is complicated. While all major commercial insurers cover TMS, the proper updated coverage policies can be difficult to find and navigate, and they have very difficult coverage criteria. This creates a confusing matrix of who is appropriate for TMS, who meets medical necessity criteria, and who has access to TMS therapy.
Several professional societies recommend TMS for adult patients with major depressive disorder who have failed to respond to one or two antidepressant medications trials (Table 1). In 2016, the Clinical TMS Society published its consensus review and treatment recommendations for TMS therapy for major depressive disorder. An expert panel of clinicians and academic researchers systematically reviewed over 100 peer-reviewed articles on TMS therapy and graded the strength of the evidence using the Levels of Evidence criteria published by the University of Oxford Centre for Evidence-Based Medicine. When the published data were deemed incomplete or insufficient, expert opinion was included as available. The results of the review and the coverage guidance recommend that left prefrontal TMS repeated daily for four to six weeks is an effective and safe treatment for depression in patients who have not responded to one or more antidepressant medications.

In 2017, “Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression” was published in the Journal of Clinical Psychiatry. Participants included a group of 17 clinicians and researchers with expertise in the clinical application of TMS, representing both the National Network of Depression Centers (NNDC) rTMS Task Group and the APA Council on Research (APA CoR) Task Force on Novel Biomarkers and Treatments. A total of 118 publications were reviewed for the consensus statement and were supplemented with expert opinion to achieve consensus on the administration of TMS for MDD in clinical settings. The expert opinion is that “TMS is appropriate as a treatment in patients with MDD even if patients are medication resistant or have significant comorbid anxiety.”
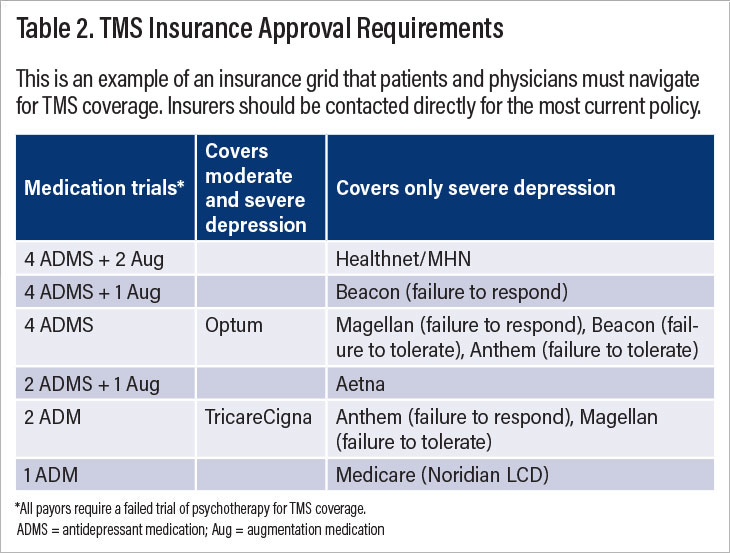
With regard to commercial payers, coverage of TMS is less uniform (Table 2). For example, Optum considers patients eligible for TMS if they failed four antidepressant medication trials and a trial of psychotherapy in the current episode, while Cigna patients are eligible for TMS if they failed two antidepressant medication trials and a course of psychotherapy. Some insurance companies require patients to fail augmentation agents such as atypical antipsychotics in addition to antidepressants, while other insurers require failure on antidepressants from different classes. Some insurers cover TMS for patients with moderate severity, while others cover TMS for patients with severe symptoms. Different insurance companies require different depression rating scales and determine eligibility for TMS based on different cutoff scores. I have had patients who are eligible for insurance coverage of TMS one month but are suddenly ineligible when their benefits change. Unfortunately, the majority of insurance policies seem to select out the most ill and treatment-resistant patients who may have attenuated benefits from TMS due to the chronicity of the depression.
Here are my recommendations on the best way to align insurance coverage policies with the standards of professional societies.
For physicians referring or prescribing TMS therapy: Prescribe or refer patients for TMS when you consider them to be good candidates. If they are denied coverage, utilize your state’s department of insurance, which should have a process for patients to appeal insurance denials.
Per society recommendations, consider TMS for patients who have moderate or severe depression and have failed one or more antidepressant medications. In my clinical practice, I start to educate patients after one failed antidepressant and prescribe TMS after two failed antidepressants.
There is only one absolute contraindication to TMS therapy—the presence of ferromagnetic metal in the head.
Align with other mental health advocacy groups and lobby your state representatives to further extend parity for mental illnesses. Legislation (SB 855) was recently signed into law in California requiring health care plans to use utilization review criteria that are consistent with the criteria and guidelines set forth by nonprofit professional societies.
For commercial insurance medical directors/medical reviewers and companies:
Meet with physicians who treat patients with TMS therapy. Consider their clinical experience when formulating coverage policies.
Attend a CME course on TMS therapy.
Review and align your company’s insurance coverage criteria with professional society guidelines. ■
“Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression” is posted here.



