Add-On Aripiprazole May Benefit Older Adults With Refractory Depression
Abstract
In a clinical trial involving older adults with treatment-resistant depression, aripiprazole augmentation was more effective than switching to bupropion, with a lower risk of falls.
For older adults with depression who have not experienced significant symptom improvements while taking antidepressants, adding aripiprazole to their existing medication regimen may offer the best chance of improving mood while limiting key side effects. These findings were based on a clinical study reported in the New England Journal of Medicine.

Though the findings point to the potential benefit of aripiprazole augmentation for older adults with refractory depression, the modest rates of remission show that more treatment options are needed, said Jordan Karp, M.D.
“From the outset, we designed this trial based on stakeholder input,” senior study author Jordan Karp, M.D., a professor and chair of psychiatry at the University of Arizona College of Medicine, told Psychiatric News. Before selecting medications and outcomes for the trial, Karp explained that the researchers surveyed psychiatrists and patients. Based on this feedback, they decided to focus the primary treatment outcome on the participants’ psychological well-being—for example, feelings of happiness, cognitive engagement, and life satisfaction. The primary safety outcomes were falls.
To try and recreate the stepwise approach typically used to treat patients with depression, Karp and colleagues designed a two-stage trial. For the first stage, which lasted approximately 10 weeks, participants were assigned to one of three groups:
One group took aripiprazole in addition to their existing medication.
Another group took bupropion in addition to their existing medication.
Lastly, one group switched from their existing medication to bupropion.
To reflect the normal circumstances under which patients receive medications, the participants in the study picked up their prescriptions from their regular clinic or pharmacy, Karp noted. Study investigators provided decision support to the participants’ primary care or mental health care provider (for example, raising or lowering the medication doses).
Any participants who did not meet the criteria for depression remission (defined as a score of 10 or less on the Montgomery–Åsberg Depression Rating Scale) after 10 weeks were eligible to enter the second stage of the study. Additionally, participants who were ineligible for stage 1 (most commonly because they had previously taken and failed to respond to aripiprazole and/or bupropion) could enroll directly into stage 2 of the trial.
A total of 619 adults aged 60 years or older entered stage 1. All participants had treatment-resistant depression, which was defined as a lack of remission following at least two attempts of antidepressant therapy (though some had tried up to eight different antidepressants); all patients were taking antidepressants at the time of enrollment. Average psychological well-being scores at baseline were 33. (The average score of the general population is 50, with higher scores indicating greater well-being.)
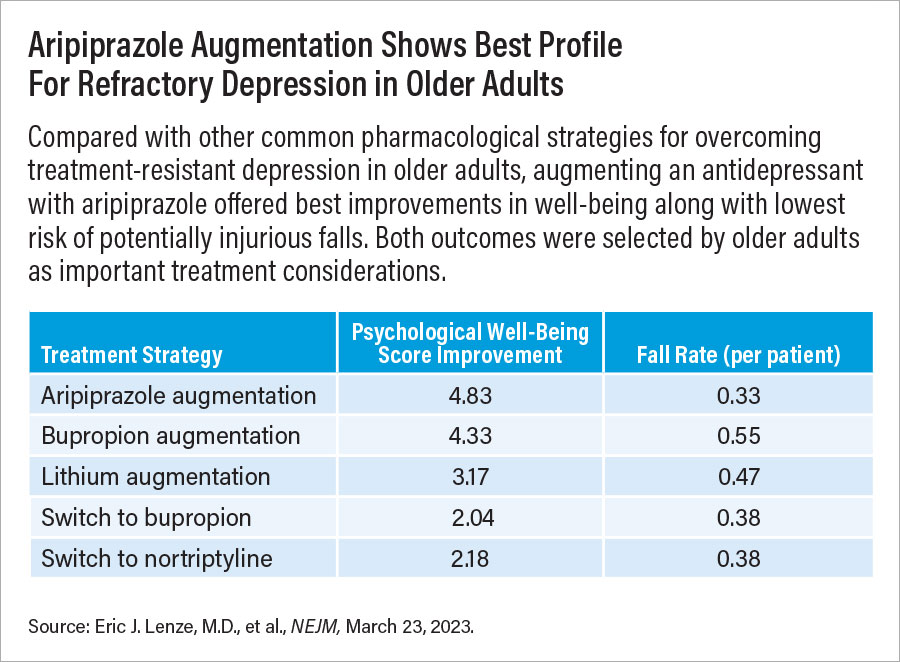
After the first stage, psychological well-being scores improved significantly more among adults who were in the aripiprazole-augmentation (+4.8 points) or bupropion-augmentation (+4.3 points) groups compared with those who switched to bupropion (+2.0 points). Remission rates were 28.9% among patients in the aripiprazole-augmentation group, 28.2% among those in the bupropion-augmentation group, and 19.3% among those who switched to bupropion.
Though patients in the aripiprazole- and bupropion-augmentation groups experienced similar improvements, bupropion augmentation was associated with a higher risk of having a fall. Overall fall rates over 10 weeks were 0.55 per patient in the bupropion-augmentation group, compared with 0.33 per patient in the aripiprazole-augmentation group and 0.38 per patient in the switch-to-bupropion group.
The second stage of the trial included 125 participants who did not achieve remission in stage 1 of the trial and 123 who enrolled directly into stage 2. These participants were given lithium in addition to their existing medication or switched from their existing medication to nortriptyline for approximately 10 weeks. (Lithium and nortriptyline are considered second-line medications because they require regular blood tests to assure safe and therapeutic plasma levels are maintained.)
After 10 weeks, the participants taking lithium in combination with an antidepressant and those who switched to nortriptyline experienced similar improvements in psychological well-being; scores rose by about 3.2 and 2.2 points, respectively. Remission rates were 18.9% among participants taking lithium in combination with an antidepressant and 21.5% among those who switched to nortriptyline. Both groups experienced similar rates of falls: 0.47 per patient in the lithium group and 0.38 per patient in the nortriptyline group.
“It is important to minimize polypharmacy in older adults, but the findings do suggest that adding aripiprazole, rather than switching drugs, offers a better chance of improvement without increasing potentially injurious falls,” Karp said. “A nearly 30% remission rate for refractory depression is encouraging.”
Still, a 30% remission rate among the participants in the aripiprazole augmentation group means that 70% of patients were still suffering, Karp continued. And on average, the participants still had psychological well-being that was less than population norms. “It speaks to how toxic depression is, how much it can impact quality of life, and the imperative that we find even more safe and effective treatments for older adults.”
This study was supported by an award from the Patient-Centered Outcomes Research Institute (PCORI). ■



