Brainwaves May Indicate Impending Depression Relapse
Abstract
With the aid of artificial intelligence and electrodes recording brainwave activity, investigators were able to discriminate signals associated with a transient change in mood from those that heralded depression relapse.
Researchers have identified a brainwave signal that might indicate if someone with depression is in stable recovery or potentially about to relapse. If validated, this biomarker could stand to significantly improve the long-term management of people receiving deep brain stimulation (DBS) for severe, treatment-resistant depression. The findings were described in a report published in Nature.
DBS is an experimental treatment for depression, which involves surgically implanting tiny electrodes (about 1.5 mm diameter) in the brain. Similar to a pacemaker, these electrodes are connected to a pulse generator that controls the stimulation delivered to the brain, modulating a circuit associated with depression.
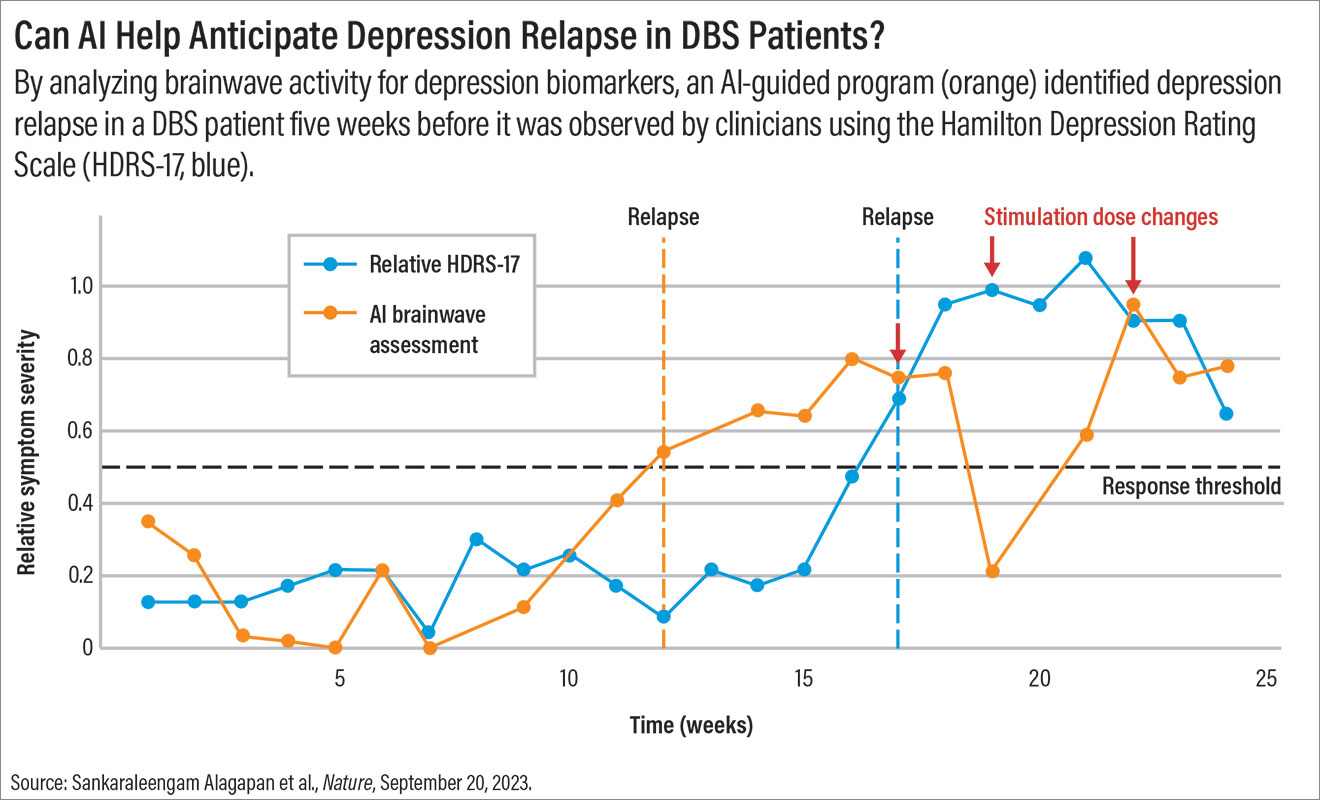
Can AI Help Anticipate Depression Relapse in DBS Patients?
With advancements in MRI tools allowing for targeted implant location, DBS has become more precise over the past decade. However, long-term clinical management is tricky because many patients receiving DBS continue to experience mood fluctuations, said Patricio Riva-Posse, M.D., an associate professor of psychiatry at Emory University in Atlanta, who was a co-author of the Nature article.
Psychiatrists like Riva-Posse rely on subjective clues about changes in their patients’ mood based on patient reports as well as their observations of the patient.
“If I notice a change in [my patient’s] mood, I have to wonder if my patient is just having a hard week or if this shift is related to the root cause of their depression,” Riva-Posse said. While the former may be treated with brief psychotherapy, a more significant shift in mood may mean a higher level of DBS stimulation is needed to prevent depression recurrence.
An objective signal to validate clinical assessments would be invaluable, he continued.
Scanning Brainwaves for Signals
As described in the Nature report, Riva-Posse and colleagues conducted DBS surgery in 10 patients with depression using a new DBS device that recorded surrounding brainwave activity. Each patient was implanted with two electrodes (one on the left and one on the right) in the subcallosal cingulate (SCC)—a brain region rich in nerve fibers that connects networks involved in mood, learning, reward, and memory formation.
“Outside of the novel device and indication, the procedure is exactly the same as standard DBS for approved indications such as Parkinson’s disease,” said Megan Frankowski, Ph.D., a program director at the National Institute of Neurological Disorders and Stroke and program officer for this project. Frankowski is also a team leader in the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, which funded this study. “There are no additional implants, and the procedure can be done with the patient either asleep or awake.”
After surgery, the electrodes remained off for 30 days while the patients recovered; stimulation was then turned on, and the participants entered a 24-week observation phase. Each week they came to the hospital for a clinical assessment and had their brainwave data briefly recorded while neural stimulation was turned off.
At 24 weeks, average depression scores, as assessed with the 17-item Hamilton Depression Rating Scale (HDRS-17), dropped from a baseline of 22.3 to 7.3. Of the 10 study participants, nine were determined to be treatment responders (50% or more decrease in HDRS-17 scores, and seven were determined to be in remission (HDRS-17 score of 8 or less). Adequate brainwave information was available for six participants: five of the patient responders and the one who experienced a relapse.
The brainwave recordings of the five patients who responded to DBS were then analyzed by a machine learning program. First, the program compared the participants’ brainwave activity at weeks 1 to 4 (when all five patients were still in a depressed state) with the participants’ brainwave activity at weeks 21 to 24 (when all five had improved). The data were used to develop a set of parameters that classified an individual as either “sick” or “stable.” The machine learning program then analyzed the recording data across all 24 weeks for each patient. As Riva-Posse explained, the software was able to differentiate brainwave signals associated with a transient change in mood from signals that required a stimulation adjustment.
The AI software was also tested on the one patient who experienced a depression relapse. In this instance, Riva-Posse noted that the software identified a shift in brain activity from stable to sick weeks before the relapse became clinically apparent; this suggests the program might be able to warn clinicians about potential problems.
Future of Neuromodulation
Alik Widge, M.D., Ph.D., an assistant professor of psychiatry at the University of Minnesota, cautioned that the data were obtained from five patients treated at a single clinical center and used an exploratory AI model where nothing was prespecified. “It’s easy to get positive conclusions that don’t hold up in larger samples,” he told Psychiatric News. But, if something like this is validated, it could be incredibly helpful.”
Widge, who was not involved with this research, added that the findings show the scientific potential of modern neural devices. “These opportunities to record the brain and study the biological basis of mental disorders … are going to let us answer questions that we just cannot [answer] with animal or computational models,” he said.
The study also examined only a few circuits from one single brain region—which he equated to “trying to view the brain through a coffee stirring straw.” However, he thinks this promising data will keep pushing the neuromodulation field toward a better understanding of the brain’s countless networks.
Frankowski agreed that this study is an important step forward for translating this currently experimental depression treatment to the clinic.
Given that researchers can develop many different AI algorithms to analyze brain signals, these devices can provide versatile information, Frankowski noted. One use currently being investigated is to track day-to-day changes in patients with obsessive compulsive disorder—another indication where DBS is promising—to see how their brain activity responds to triggering situations in the real world as opposed to a doctor’s office.
“That’s an important part of the mission of the BRAIN Initiative,” she said. “We strive to support projects that not only develop new tools to better understand the brain, but also develop new and improved therapies for brain disorders.”
This study was supported with grants from the NIH BRAIN Initiative and the James S. McDonnell Foundation, along with support from the Hope for Depression Research Foundation, National Science Foundation, and the Julian T. Hightower Chair at Georgia Tech. The Activa PC+S devices were provided by Medtronic. ■



