Drug Companies Sue to Overturn State Medicaid Programs
The legal challenge to the use of preferred drug lists for Medicaid patients has moved to the federal courts.
On August 28 the Pharmaceutical Research and Manufacturers of America (PhRMA) presented oral arguments in the U.S. District Court in Washington, D.C., in its lawsuit challenging a Medicaid preferred drug program implemented last February in Michigan (Psychiatric News, August 2, June 21, April 19, January 18).
PhRMA filed suit against Tommy Thompson, secretary of the Department of Health and Human Services, and Thomas Scully, administrator for the Centers for Medicare and Medicaid Services, in their official capacities. The association asked for a preliminary injunction invalidating the program, which is already under way.
The Michigan program is budgeted to generate $42 million annually by requiring pharmaceutical companies to give the state supplemental rebates on the cost of drugs to have their products on a preferred drug list that does not require prior authorization.
Under the program, pharmaceutical companies are required to agree to rebates so that the state’s net payment for the drug equals the lowest price charged in the United States for any drug in the same broadly defined therapeutic class.
PhRMA lawyers alleged that Michigan is violating federal law by excluding drugs from the list solely on the basis of price. Federal law had already established requirements for rebates, which result in an average rebate of 15.1 percent of the average manufacturers’ price of the drug.
The lawyers also maintained that it is unconstitutional to use out-of-state pricing benchmarks to determine state rebates.
According to PhRMA, Michigan has no legal grounds to require that pharmaceutical companies offer rebates to states for non-Medicaid programs. The result, the lawyers argued, is that Medicaid patients could be hurt by actions to secure savings in non-Medicaid programs.
Federal law stipulates that states can exclude drugs from formularies only if they do not have “significant, clinically meaningful therapeutic advantage in terms of safety, effectiveness, or clinical outcomes.”
State lawyers in Florida and Michigan have maintained that a preferred drug list is different from a formulary because a preferred drug list permits a drug to become available with prior authorization.
The National Alliance for the Mentally Ill of Michigan, the National Urban Indian Coalition, the Florida Drop-In Center, and the International Patient Advocacy Association jointly filed a friend-of-the-court brief supporting PhRMA’s position.
State Challenge Continues
Four advocacy organizations filed suit in December 2001 to stop implementation of the Michigan program.
In a January 9 ruling, Judge Lawrence Glazer of the Ingham County Circuit Court issued an injunction to the program and supported an argument that the program would cause irreparable harm to patients.
He wrote, “The system of telephone appeals to a technician and then to a pharmacist and then to a physician, only during business hours, will undoubtedly result in delays in the dispensing of the medications [that] physicians judge to be medically necessary. . . .”
The injunction was lifted on January 18 by a ruling of the Michigan Court of Appeals, but that court did not consider the merits of the case. On September 5 the Court of Appeals heard arguments and has not yet rendered a decision.
What’s at Stake
According to the PhRMA written complaint, Medicaid prescription drug sales nationwide total approximately $20 billion each year. Those drugs have become a target for cost cutting because of declining state revenues and accelerating Medicaid costs.
According to the National Governors Association, Medicaid accounts for an average of 20 percent of state budgets. Costs of the program have grown in both absolute and relative terms in recent years. As a result, Medicaid is the second-largest expenditure in most state budgets.
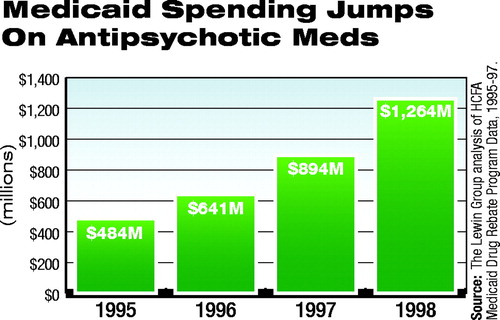 The Urban Institute estimated that Medicaid spending for outpatient prescription drugs increased by an average of 18.1 percent a year from 1997 to 2000, compared with 7.7 percent for all Medicaid expenditures (see chart for increased expenditures on antipsychotic medications).
The Urban Institute estimated that Medicaid spending for outpatient prescription drugs increased by an average of 18.1 percent a year from 1997 to 2000, compared with 7.7 percent for all Medicaid expenditures (see chart for increased expenditures on antipsychotic medications).
The July 15 New York Times reported that 45 states claimed revenue shortfalls over the last year totaling $50 billion, caused by a drop in sales, capital gains, and corporate and personal income taxes.
States that have initiated or announced programs that, according to PhRMA, share “some or all of the illegal characteristics of the Michigan program” include Connecticut, Florida, Hawaii, Illinois, Louisiana, Minnesota, Mississippi, Missouri, New Mexico, North Carolina, Ohio, Vermont, and West Virginia.
The July/August 2002 issue of Psychiatric Practice and Managed Care contains talking points concerning psychotropic medications and APA’s Position Statement on Pharmacy Benefit Management. A supporting resource document can be obtained on the Web at www.psych.org/archives/200207.pdf or by phone at (888) 357-7924. The June/July issue of APA’s State Update features issues related to Medicaid drugs. It is posted at www.psych.org/pub_pol_adv/su_junejuly72602.pdf. PhRMA’s federal complaint and related documents are posted at www.phrma.org/litigation. ▪



