Soaring Internet Drug Sales Raise Safety, Legal Concerns
Type in “Canada prescription drugs” using any standard computer search engine, and within seconds your screen will be filled with the names of companies that ship prescription drugs to the United States from Canadian pharmacies.
In fact, according to the April AARP Bulletin, an estimated 80 to 90 Web sites now offer to sell prescription drugs via mail order, and an estimated 1 million Americans use this cross-border pipeline.
The Web site www.kaisernetwork.org reported on April 22 a prediction from the business analysis firm Jupiter Research that Canada’s Internet drug market will double in 2003 to $1.4 billion and that the $700 million in reimported drugs purchased last year cut profits for American drug companies by $1.3 billion, or about 1 percent of the U.S. retail pharmaceutical market.
 The reason for the popularity of this method of mail-order shopping is not hard to discover. Pharmaceutical prices are usually considerably lower in Canada than in the United States. Among the lowest Internet prices are those offered by the Minnesota Senior Federation (MSF). Staff of the nonprofit organization negotiated with a licensed Toronto pharmacy to cut its professional fees to provide even deeper discounts.
The reason for the popularity of this method of mail-order shopping is not hard to discover. Pharmaceutical prices are usually considerably lower in Canada than in the United States. Among the lowest Internet prices are those offered by the Minnesota Senior Federation (MSF). Staff of the nonprofit organization negotiated with a licensed Toronto pharmacy to cut its professional fees to provide even deeper discounts.
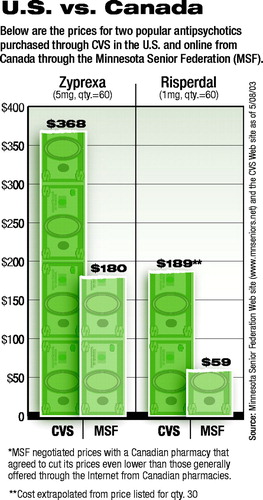
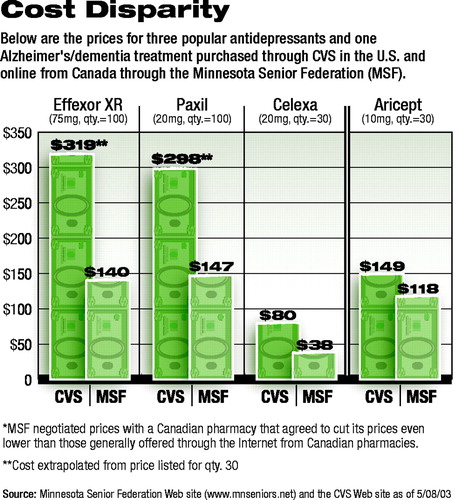 The accompanying chart shows MSF prices for psychotropic medications compared with those listed at the CVS Pharmacy Web site. With the exception of Aricept, all prices through MSF are 50 percent or less than those charged by CVS.
The accompanying chart shows MSF prices for psychotropic medications compared with those listed at the CVS Pharmacy Web site. With the exception of Aricept, all prices through MSF are 50 percent or less than those charged by CVS.
A $19 membership is required to order through the Canadian Prescription Drug Importation Program, operated by MSF. The organization posted a letter for physicians describing the program at its Web site.
The letter explains that the MSF is serving as an intermediary between its members and Canada RX, an exporter of prescription drugs. Participating physicians fill out the physician export order form, which requires information on the name of medication, strength, quantity, whether generic substitution is allowed, and the number of refills.
Physicians must also fill in their name, address, DEA number, license number, and information on where the prescription is to be shipped. The patient faxes that form and the prescription itself to Canada RX.
No medical information from the patient or physician is required on the forms.
According to the AARP Bulletin, “reputable [online Canadian] pharmacies” display the pharmacy’s license number and name of the authority that granted it. They require patients to provide a doctor’s prescription, which will be reviewed by a Canadian doctor as required by Canadian law.
The pharmacies require patients to submit details of their medical history and provide a telephone number so patients can speak with a pharmacist or ask questions about the service.
Safety of the drugs and legality of reimportation are the two problems raised by virtually all opponents of the Internet prescription drug programs.
In September 2002, the Pharmaceutical Research and Manufacturers of America (PhRMA) announced an ad campaign that challenged the safety of reimportation.
The ad read, in part, “U.S. health and safety officials from the Department of Health and Human Services, the Food and Drug Administration (FDA), the Customs Service, and the Drug Enforcement Agency have warned Congress that reimportation could open America’s medicine cabinet to an influx of dangerous drugs.”
In a “Faceoff” article published in the May AARP Bulletin, Peter Wyckoff, MSF’s executive director, wrote, “The U.S. Food and Drug Administration obviously cannot guarantee the safety of medications purchased in Canada. Rather, consumers rely on the oversight of the Canadian Ministry of Health, which assumes its responsibility as well as the FDA. . . . Drugs manufactured for the Canadian market come from the same FDA-approved facilities as those destined for the American market.”
He told Psychiatric News, “We don’t want to be in the drug-reimportation business. We would much prefer that policies and laws change so that U.S. citizens have access to drugs in their own country at the same prices paid by people in countries around the world.”
According to the May 8 Washington Post, the Canadian government said officially that it would be responsible for the safety and quality of drugs imported to the United States. Daniele Dionne, Health Canada’s associate director general, described the statement as a “clarification,” rather than a new policy.
FDA Commissioner Mark McClellan, who worked with Canadian officials on the statement, told the Post reporter, “The fact that they are explicitly stating that they are trying to assure safety and effectiveness not only for Canadians, but for the millions of prescriptions sold to Americans through Canada, is a potentially useful step.”
In late May, however, the Post reported that the FDA said it had misunderstood Canada’s position on prescription-drug safety. Instead of expanding oversight of these medications, Canada’s position is that while it regulates all imported prescription drugs, it cannot guarantee their safety when they are reimported into the United States.
According to the March 12 Wall Street Journal, in a letter dated February 12 to Robert P. Lombardi, a lawyer representing health plans, William K. Hubbard, the FDA associate commissioner for policy and planning, wrote, “[All parties] who cause a prohibited act” can “be found civilly and criminally liable” under the Food, Drug, and Cosmetic Act.
“Those who aid and abet a criminal violation of the act, or conspire to violate the act, can also be found criminally liable.” The letter added, “Any party participating in an import plan in which a health insurer or claims processor helps arrange a purchase in Canada does so at its own legal risk.”
The Wall Street Journal reported on March 24 that the FDA had sent a warning letter to RxDepot Inc. of Tulsa, Okla., charging that it was in violation of the Food, Drug, and Cosmetic Act. RxDepot operates by sending U.S. prescriptions to Canada, where they are rewritten and the drugs shipped.
On April 4 the Wall Street Journal reported on a hearing of the House Subcommittee on Human Rights and Wellness in which Rep. Dan Burton (R-Ind.) and Rep. Bernard Sanders (I-Vt.) charged that the FDA had no evidence of a safety problem and was frightening seniors.
Hubbard argued for the FDA that importation would increase the likelihood that counterfeit and substandard drugs would enter the U.S. and that the FDA is focusing its efforts on businesses, not individuals, who are purchasing drugs.
APA has taken no position on reimportation of drugs, and individual members are responding to the issues involved in different ways.
Anthony D’Agostino, M.D., told Psychiatric News that he is concerned about the underlying problems concerning access to drugs that have resulted in their reimportation from Canada. He is medical director of Alexian Brothers Behavioral Health Hospital in Hoffman Estates, Ill., and the chair of the Subcommittee on Pharmacy Benefits Management of APA’s Managed Care Committee.
“One of my patients has such a limited income that she can’t even get in to see me unless she gets a ride,” he said. “When she asked me to fill out a form for her so she could use an Internet site and save money on her prescription, I did.”
Galen Stahle, M.D., who is in private practice in Deephaven, Minn., is taking a more aggressive position. “Prices are out of control,” he told Psychiatric News.
He supports the work of MSF and distributes to his patients a list of selected U.S. and Canadian Internet sites where they can purchase prescription drugs.
Stahle also protested to GlaxoSmithKline (GSK) when the company announced in January 2003 that it would no longer sell products to Canadian pharmacies and wholesalers that market GSK drugs over the Internet to other countries.
“I wrote to Jean-Pierre Garnier, GSK’s CEO for U.S. operations, and said GSK’s drug reps would not be welcome in my office unless the company changed its policy,” he said.
According to the Web site www.kaisernetwork.org (April 22), GSK now is asking companies to “self-certify” that they are not exporting the company’s drugs. As of press time, GSK had not responded to a request for a comment.
Stahle brought the issue of reimportation to the Private Care Committee of the Minnesota Psychiatric Society (MPS). MPS President Karen Dickson, M.D., told Psychiatric News that MPS needs more information before deciding whether to take a position.
“We are concerned about how price affects access to drugs that our patients need,” she said, “but we can’t be in the position of recommending or encouraging a practice that is illegal or unsafe.”
According to the May 11 Detroit News, the Michigan State Medical Society agreed to study the issue of buying drugs in Canada after members of the Oakland County and Kent County medical societies introduced resolutions on the issue at their annual delegates’ meeting.
AMA president Yank D. Coble Jr., M.D., issued a statement on March 24 applauding “the warning to an Internet prescription service by the FDA. The FDA warned RxDepot that it considers the firm’s operation illegal and a risk to public health. The AMA remains vigorously opposed to the illegal sale of prescription drugs via the Internet.”
A spokesperson for the AMA told Psychiatric News that the issue of reimportation will be discussed at the meeting this month in Chicago of AMA’s House of Delegates. ▪



