Maine to Work With HHS After Court Allows Medicaid Drug Plan to Operate
On May 19 the U.S. Supreme Court issued a 6-3 decision that lifted an injunction preventing the Maine Rx program from being implemented.
Gov. John E. Baldacci (D) convened a work group of legislators and health advocates who helped design the original drug program to examine the decision and revise the program if necessary. On May 29 he presented a revised prescription drug program, Maine Rx Plus, to the legislature.
Maine Rx Plus calls for the establishment of a preferred drug list for participants in MaineCare (the state’s Medicaid program). Residents eligible for Maine Rx Plus will be able to buy the drugs on the MaineCare preferred drug list at Medicaid prices, which are discounted.
The new program specifies that eligibility for Maine Rx Plus is limited to those whose income is at 350 percent of the federal poverty level or lower. The income limit for a family of four will be approximately $64,400. Maine Rx did not have an income restriction.
In the Supreme Court decision, Justices Stephen G. Breyer and John Paul Stevens noted that the secretary of Health and Human Services (HHS) has the legal right to review Maine Rx because the program could be viewed as an amendment to the state’s Medicaid plan, which requires HHS approval for implementation.
In an opinion joined by Justice David Souter, Justice Ruth Ginsburg, and Breyer, Stevens wrote, “[W]e offer no view as to whether it would be appropriate for the Secretary to disapprove this program if Maine had asked the Secretary to review it. We also offer no view as to whether it would be proper for the Secretary to disallow funding for the Maine Medicaid program if Maine fails to seek approval from the Secretary of its Maine Rx Program.”
A spokesperson for Maine’s Department of Human Services told Psychiatric News that state officials planned to work with HHS to find “common ground” concerning the program.
The court rejected the argument of the Pharmaceutical Research and Manufacturers of America (PhRMA) that the plan discriminates against interstate commerce in order to subsidize in-state retail sales. Stevens wrote, “Maine Rx does not regulate the price of any out-of-state transaction by its express terms or its inevitable effect. Nor does Maine Rx impose a disparate burden on out-of-state competitors.”
Stevens, joined by Souter and Ginsburg, ruled against the argument that the program violated federal law because it might interfere with Medicaid benefits without serving any Medicaid purpose.
He wrote that the program might serve the Medicaid-related purposes of providing benefits to “needy people” and curtailing the state’s Medicaid costs. With access to less-expensive drugs, people with low incomes might not end up on the Medicaid rolls.
Stevens wrote, “The Medicaid Act gives states substantial discretion to choose the proper mix of amount, scope, and duration limitations on coverage as long as care and services are provided in the recipients’ best interests.”
Maine’s legislature enacted the program in 2000. In October of that year, PhRMA successfully challenged the program in the U.S. District Court for Maine based on constitutional grounds, including violations of the interstate commerce clause (Psychiatric News, February 1, 2002).
 Maine appealed to the 1st Circuit Court of Appeals in Boston. The court ruled in the state’s favor, saying that the program does not “regulate” the drug companies’ interstate commerce and that the effect on the drug industry is not excessive in light of the benefits to be obtained. PhRMA then appealed the case to the U.S. Supreme Court.
Maine appealed to the 1st Circuit Court of Appeals in Boston. The court ruled in the state’s favor, saying that the program does not “regulate” the drug companies’ interstate commerce and that the effect on the drug industry is not excessive in light of the benefits to be obtained. PhRMA then appealed the case to the U.S. Supreme Court.
In a press release about the Supreme Court decision, PhRMA spokesperson Marjorie Powell said that the association was reviewing the court’s “complex, multifaceted opinions.”
She continued, “As we review the ruling, we note that Justice Stevens’ opinion makes clear that today’s decision does not ‘determine the validity of the Maine program.’ The key issues have yet to be decided by the District Court (where the case was returned to weigh evidence about the burdens and benefits of the program for Medicaid patients).”
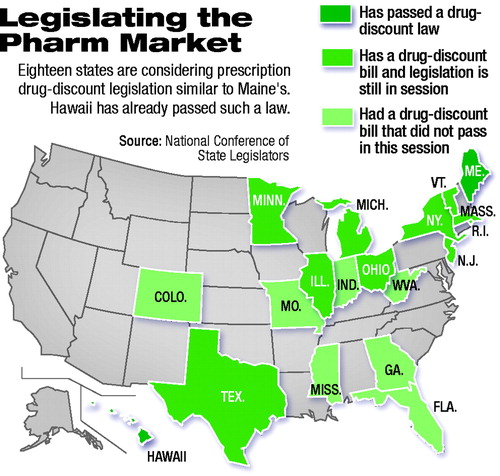
According to the National Conference of State Legislators, legislators in 18 states have offered bills to create drug-discount programs similar to that in Maine (see map).
The Supreme Court decision in Pharmaceutical Research and Manufacturers of America v. Walsh, Acting Commissioner, Maine Department of Human Services, et al., No 01-188, is posted on the Web at http://laws.findlaw.com/us/000/01-188.html. Information about the decision and related state initiatives is posted at www.ncsl.org/programs/health/pharm.htm#b. ▪



