Seizure Drug Combats Antipsychotic Side Effects
The metabolic complications sometimes associated with the use of some second-generation antipsychotics (SGAs)—changes in weight, serum lipids, triglycerides, and glucose—have become increasingly worrisome to clinicians managing patients with serious mental illness. Now, physicians and patients may have an unexpected ally in the fight against these potentially serious effects.
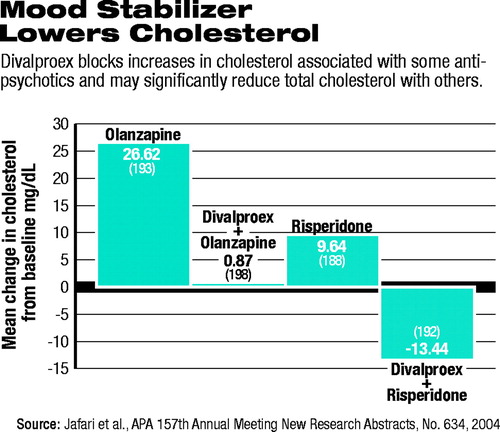
New research presented at APA's annual meeting last month confirms earlier reports that divalproex sodium (Depakote) blocks the increases in serum cholesterol often seen with two of the most often prescribed SGAs, olanzapine (Zyprexa) and risperidone (Risperdal). Those changes in cholesterol have been strongly associated with cardiovascular disease. Studies now show that cardiovascular disease is a leading cause of death among patients with schizophrenia.
“This really involves a recalculation—a recalibration of psychiatrists' way of practicing,” Daniel Casey, M.D., told Psychiatric News. “We must learn to think below the neck again—we did it in medical school, and we must do it in practice. We need to understand metabolic syndrome and its consequences so that we can safely manage our patients.”
Casey is a professor of psychiatry and neurology and associate director for the Mental Illness Research, Education, and Clinical Center at Oregon Health and Sciences University in Portland.
Casey's research was presented as a poster at the annual meeting. The research was funded by Abbott Laboratories, maker of the Depakote brand of divalproex, approved 20 years ago as an antiepileptic. The drug is considered first-line therapy (along with lithium) for mania in the APA Practice Guideline for the Treatment of Patients With Bipolar Disorder, having gained an indication for the treatment of acute manic episodes associated with bipolar disorder in 1996. (Divalproex is also approved for the prevention of migraine.)
“Our patients are at high risk for metabolic disorders—both from a possible increased predisposition that could be genetically based, but also largely as a consequence of the medications we prescribe,” Casey noted. “It is inherently important for psychiatrists to do necessary primary care follow-up. Our patients are usually more in contact with us than with any other physician.”
All physicians play a part in spotting the risk of potentially fatal metabolic syndrome in their patients, Casey added. “Some will serve as identifiers, some as evaluators, and some as treating physicians. All psychiatrists must at least be an identifier.”
The meaning of the term “metabolic syndrome” varies from one source to another, but most commonly describes a clinical picture that begins with weight gain associated with elevations in serum cholesterol and triglycerides and is accompanied over time with an increase in resistance to insulin. If insulin resistance goes untreated, patients may develop diabetes, in addition to cardiovascular disease. The origins and mechanisms of metabolic syndrome are not yet understood, but it is clear that morbidity and mortality are markedly increased.
Casey noted that psychiatry is certainly making progress in dealing with the syndrome, having released the joint APA/American Diabetes Association consensus guidelines on appropriate monitoring of patients on antipsychotics (Psychiatric News, March 5). APA's Office of Research and Council on Research are also formulating formal APA guidelines on the issue.
“You have to have some sort of consensus on what really is the problem and how to deal with it,” Casey said. He noted that it is hard to get insurance coverage for metabolic monitoring and testing of psychiatric patients, but he thinks that will soon change.
“Once you have formal guidelines, people start looking at liability and risk management. They will look at the consequences and the associated costs of managing [these problems] against those of not monitoring.”
Casey's study was a post hoc analysis of a double-blind, randomized, multicenter trial that assessed the efficacy and safety of divalproex when combined with either risperidone or olanzapine as adjunctive therapy for schizophrenia.
The study randomly assigned 249 patients to olanzapine monotherapy, olanzapine plus divalproex, risperidone monotherapy, or risperidone plus divalproex.
Patients in the olanzapine monotherapy group had the highest rate of shift from a normal total cholesterol (less than 200 mg/dl) to a high total cholesterol (above 200 mg/dl). Nearly 55 percent of patients taking olanzapine alone shifted from normal to high cholesterol, with an average increase in cholesterol of 27 mg/dl. Of those patients taking olanzapine and divalproex, only 27 percent saw an increase to a total cholesterol over 200 mg/dl, and the average increase was much smaller, less than 1 mg/dl.
Those patients randomly assigned to risperidone alone had a moderate rate of shifting from normal to high cholesterol as well, although not as high as that of the patients taking olanzapine. Twenty seven percent of patients shifted, with an average increase of about 10 mg/dl. Patients taking both risperidone and divalproex saw dramatically different changes. While about 5 percent of these patients shifted from normal to high cholesterol levels, the majority of the group experienced an average decrease in total cholesterol of 13.5 mg/dl.
Interestingly, no significant correlations were seen between baseline weight, body-mass index, and total cholesterol. Also, serum levels of olanzapine and risperidone were not affected by the addition of divalproex, and there were no significant differences in glucose levels in the four groups.
“Across the categories, in this study and in the previous research,” Casey emphasized, “there has been a fairly consistent 25 percent reduction of cholesterol in patients taking the combination compared with the antipsychotic monotherapy.” These data, he noted, are consistent with data on patients with bipolar disorder and patients with epilepsy given divalproex.
Casey is not sure of the mechanism by which divalproex lowers serum cholesterol; however, he and other researchers suspect that the drug is altering the liver's metabolism of cholesterol.
The results are peculiarly interesting because divalproex's effects on the liver are well known. For years the drug has been associated with very rare cases of drug-emergent liver failure, sometimes requiring transplant or resulting in death. In addition, the drug has been associated with rare reports of pancreatitis. In this study, Casey noted, divalproex ameliorated the increase in liver enzymes that were observed in the olanzapine and risperidone monotherapy groups, suggesting an interactive protective effect on the liver. Pancreatic enzymes were not monitored in this study.
“As psychiatrists, we have a high-risk population, and our choice of medication can profoundly affect someone's chance of converting from borderline to clearly abnormal [metabolic indicators],” Casey stressed.
Studies of the general population have shown that a 10 percent reduction in cholesterol can result in a 20 percent to 30 percent reduction in the risk of cardiovascular disease, Casey noted. He hopes attenuating increases in cholesterol tied to SGAs by prescribing divalproex will lead to the same benefit. ▪



