Med Check
Regulatory & Legal Briefs
Duloxetine, Eli Lilly's new combined selective serotonin-norepinephrine reuptake inhibitor to treat major depression received final approval (Original article: see article at right). | |||||
Acamprosate, Merck KGaA's new treatment for the prevention of relapse in alcoholism received final approval. It will be distributed in the U.S. by Lipha Pharmaceuticals, a subsidiary of German conglomerate Merck (see article on Original article: page 30). | |||||
Quetiapine received Food and Drug Administration (FDA) approval for the treatment of mania associated with bipolar disorder, with the addition to the label of data from studies that demonstrated the drug's safety and efficacy beyond the treatment of acute mania. Data from two identical randomized controlled trials involving 599 patients showed that after 12 weeks, 65.4 percent of patients receiving quetiapine achieved remission (a Young Mania Rating Scale score of less than or equal to 12) compared with 35.9 percent of those receiving placebo. | |||||
Gepirone ER, an investigational 5-HT1A agonist available in Europe, was found to be not approvable by the FDA for treatment of major depressive disorder. The FDA determination was based on an amendment to the gepirone new-drug application that was submitted in December 2003. Dutch pharmaceutical company Organon announced its intention to withdraw the application and “move forward with our next steps in the development of other products for mental health,” a company statement said. | |||||
The FDA has accepted a resubmission of the approval application for eszopiclone, Sepracor's new hypnotic. The agency issued an approvable letter on February 27 and cited issues that needed to be addressed by the company prior to final approval. No additional clinical studies or data analyses were requested. The FDA has classified the review of eszopiclone as a Class 2, meaning the agency expects to complete the review and issue a final determination within six months. The new “sleeper” will be indicated for the treatment of insomnia characterized by difficulty falling asleep and/or difficulty maintaining sleep during the night. | |||||
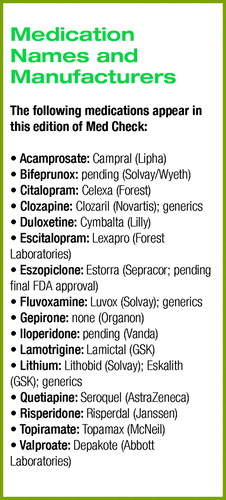
Research Briefs
Escitalopram shows consistently better efficacy in treating major depression than its parent drug, citalopram, because the R-enantiomer present in citalopram competes with and counteracts the activity of the S-enantiomer. Escitalopram, which contains only the therapeutically active S-enantiomer, avoids this conflict, according to a review of a large body of research. Data from randomized controlled trials have not only shown escitalopram to be associated with higher rates of response and remission compared with citalopram, but patients taking escitalopram achieve remission significantly faster than those taking citalopram. In addition, escitalopram is largely free of drug-to-drug interactions and has a minimal side-effect profile. Psychopharmacol 2004; 174:163-176 | |||||
Lamotrigine, which has been shown in clinical trials to be effective in treating both mania and depression associated with bipolar disorder, modulates numerous channels in the cell membranes of neurons, potentially stabilizing the cells' excitability. In particular, research now speculates that the efficacy of the drug in bipolar disorder may be due to its ability to modulate use-dependent—or selective—inhibition of neuronal firing, potentially leading to an attenuation of overfiring associated with mania. Lamotrigine also inhibits glutamate (as does lithium) but does not decrease the expression of protein kinase C (as does valproate). J Clin Psychiatry 2004; 65:791-804 | |||||
Fluvoxamine appears to attenuate weight gain and metabolic disturbances commonly seen in patients taking clozapine. In a study of 68 treatment-resistant inpatients with schizophrenia, patients were randomly assigned to either clozapine alone or low-dose clozapine (less than 250 mg per day) plus fluvoxamine (50 mg per day). After 12 weeks of therapy, patients taking both drugs had significantly lower body weight, body mass index, and serum levels of glucose and triglycerides. Changes appeared to correlate with patients' blood levels of norclozapine, a normal metabolite of clozapine, but not blood levels of clozapine itself. In patients taking both drugs, norclozapine levels were significantly lower compared with patients taking clozapine alone, suggesting that fluvoxamine altered the metabolism of clozapine and thereby blocked the adverse effects of norclozapine. J Clin Psychiatry 2004; 65:766-771 | |||||
Quetiapine has been associated with at least 69 reports of drug-associated hyperglycemia or diabetes. Between January 1, 1997 and July 31, 2002, there were 46 such reports. Patients with new-onset diabetes following initiation of quetiapine therapy tended to be younger than those whose pre-existing diabetes was exacerbated by quetiapine. Overall, there were nearly two male reports for each female report. Most cases appeared within six months of starting quetiapine, and the severity ranged from mild glucose intolerance to diabetic ketoacidosis or coma. There were 21 cases of DKA and 11 deaths. An additional 23 cases were identified between August 1, 2002 and November 30, 2003, after the initial study period but prior to publication. J Clin Psychiatry 2004; 65:857-863 | |||||
Risperidone appears to be associated with more weight gain—as a percentage of baseline body mass—during the preadolescent years than later years, and weight gain appears to decrease with advancing age. This was found to be true even though preadolescent youths and adolescents received lower mg/kg/d doses than adults or the elderly. The results are based on a systematic review of literature, including double-blind clinical trials, open-label extension studies, and case series. The results suggest that regardless of dosing, youths are the most sensitive to antipsychotic-induced weight gain. J Clin Psychopharmacol 2004; 24:429-436 | |||||
Topiramate appears to improve self-reported measures of drinking in patients with alcohol dependence, a finding corroborated by blood analyses in a new study of 150 patients. For 12 weeks, half the patients received topiramate (escalating from 25mg/day to 300mg/day), and half received placebo. All patients participated in a minimal intervention called the Brief Behavioral Compliance Enhancement Treatment. Self-report measures of drinking and blood levels of serum gamma-glutamyl transferase—a sensitive marker of heavy alcohol intake—were collected at baseline and at three, six, nine, and 12 weeks. In addition to reductions in reported drinking, topiramate was associated with a decrease in patients' levels of alcohol craving. Side effects reported in the study included dizziness, tingling of the skin, psychomotor slowing, word-naming difficulties, and weight loss. Alcohol Clin Exp Res 2004; 28:1137-1144 | |||||
Industry Briefs
Titan Pharmaceuticals announced that continued development of iloperidone will be undertaken by Vanda Pharmaceuticals. Vanda has acquired the worldwide development rights to the investigational antipsychotic from Novartis. Vanda will complete phase 3 testing of the drug. | |||||
Bifeprunox, the investigational third-generation antipsychotic being developed by Solvay and Wyeth, could easily reach sales of more than $1 billion by 2008 or 2009, according to company analysts. The partial dopamine agonist is associated to date with little or no weight gain, no dysregulation of prolactin, lipids, or glucose, no prolongation of the QTc interval, and a favorable extrapyramidal-side-effect profile. The drug is currently in an extensive late phase 3 clinical program. Solvay and Wyeth plan to submit the drug for approval in 2006 with an expected market launch in 2007. ▪ | |||||



