CMS Acknowledges Need for Inclusive Part D Formulary
APA has commended the Centers for Medicare and Medicaid Services (CMS) for“ recognizing the medication needs of the particularly vulnerable population our members serve.” The Association continues to be concerned, however, over some drug utilization management techniques and the potentially discriminatory restrictions those techniques may impose on patients' access to medications in the Medicare Part D prescription drug benefit.
The praise and concern were part of written comments submitted to CMS on March 6 in response to a draft guidance outlining how the agency plans to review drug formularies submitted by prescription drug plans (PDPs) that intend to participate in the program in 2007.
In that draft guidance, issued February 23, CMS proposed to continue the 2006 requirement that all PDPs include in their Part D formularies “all or substantially all” medications in six specific classes, including antidepressants, antipsychotics, and anticonvulsants.
In response, APA “strongly endorsed” the continued protection for the six classes of medications, which also include the immunosuppressants, antiretrovirals, and antineoplastics.
“Denial of the medications in these classes would not only harm enrollees,” APA told CMS, “but in the long run would be detrimental to the entire Medicare program because of [potential] expenses incurred in increased hospitalizations and other acute care services.”
Moreover, APA strongly recommended that the agency add several protective steps to its review process for formularies, in addition to the core requirements outlined in the United States Pharmacopeia (USP) Model Guidelines.
The USP's Model Guidelines were developed in 2004 as an outline of the drug categories, classes, and key drug types that should be included in formularies of all PDPs participating in Part D (Psychiatric News, October 1, 2004). Under the Medicare Modernization Act, which created the Part D benefit, all PDPs participating in Part D must cover at least two medications“ within each therapeutic category and class of covered Part D drugs, although not necessarily all drugs within such classes.”
In the comments that APA submitted to CMS, APA stated that the final guidance should refer to treatment guidelines that outline best practices to guide PDPs in developing their 2007 formularies. PDPs could, for example, review the practice guidelines developed by APA, the Texas Medication Algorithm Project, and the Schizophrenia Patient Outcomes Research Team. These expert guidelines, APA noted, could be valuable in alleviating problems experienced with some PDPs' denial of certain medications or particular dosing regimens for specific illnesses.
In addition, APA continued, “it is our view that many widely used pharmacy management practices are wholly inappropriate if applied to medically vulnerable populations.... Best practices for the Part D patient population served by APA members have been developed and should be CMS's reference point.”
Safety Edits Present Problem
APA is particularly concerned with the current guidelines' use of“ so-called safety edits by PDPs to establish quantity limits.” The Association commends the use of such edits when they serve as Drug Utilization Review alerts, that is, requiring verification from the physician of, for example, a dose of medication prescribed that is outside usual parameters. APA is concerned, however, that “[u]nder Part D thus far in 2006, safety edits are being used to restrict access to necessary doses of medications, even when the physician assures the PDP that the patient has been stabilized on the dose prescribed.”
APA also expressed concern over language in the draft guidance that would, for example, appear to allow step therapy or “fail-first” utilization management techniques, as well as language that appears to allow formularies to exclude extended-release formulations when immediate-release versions of the same medication are covered.
Finally, APA told CMS, it is working “with other expert groups” to develop a detailed analysis of issues “inherent in the use of conventional utilization techniques for patients with mental illness.” APA welcomes “the opportunity to dialog with CMS about this activity and hope[s] that [APA and CMS] can develop specific guidelines that will aid CMS in its review of PDP utilization management practices.”
APA Objects to USP Proposal
APA's latest round of comments came two months after the Association had submitted written comments to the USP regarding a proposed revision of its Model Guidelines for 2007 for drug categories and classes covered in Part D formularies. In those comments, signed by APA President Steven Sharfstein, M.D., APA said that without the addition of specific protections, the proposed revision would likely lead to decreased access to psychiatric medications for Medicare beneficiaries with mental illness and result in substandard clinical care.
“The proposed guidelines,” Sharfstein wrote, “do not provide an appropriate drug classification system for beneficiaries with mental illnesses and substance use disorders. The specific therapeutic categories involved are antidementia agents, antidepressants, antipsychotics, and bipolar agents. Moreover, the complete absence of a therapeutic category for addiction treatment agents is inexplicable” (see story below).
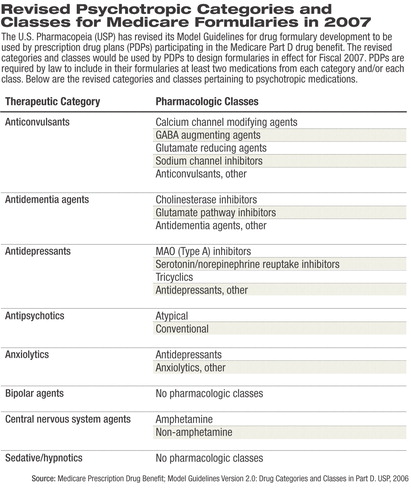
Not only are the proposed model guidelines inconsistent with current standards of care, the proposed categories and classes (see table at right) compress the available medications in a manner that is “inconsistent with appropriate organizing principles for these drugs and inconsistent with accepted practice in the field.”
Sharfstein told USP that the “potential chilling effect of the current guidelines on beneficiary access and physician treatment options” had been partially ameliorated [during 2006] by CMS's adoption of the “all or substantially all” formulary guidance. However, he cautioned, “subregulatory guidance should not and cannot be a substitute for an appropriate classification system.”
Sharfstein noted that APA's review of the original model guidelines“ found them insufficient to support necessary access to medications....”
“APA believes that the proposed revisions to the USP Model Guidelines... have deficiencies that could harm beneficiaries' access to needed drugs,” he wrote.
Last year CMS issued a subregulatory guidance aimed at clarifying how the agency would determine whether formularies submitted by PDPs included all the drugs required by the USP Model Guidelines. (A guidance is an interpretative document that can be changed by a federal agency without going through the formal regulatory process.)
The USP Model Guidelines for 2006 listed 146 drug categories, classes, and key drug types; however, psychotropic medications were distilled into categories and classes that APA believed would promote PDPs' coverage of only two of the least-expensive antidepressants or antipsychotics. In addition, coverage of medications for substance abuse appeared to be absent.
Partly in response to input from APA and allied organizations, CMS adopted the “all or substantially all” requirement to ensure access to specific medications. The February 23 CMS draft guidance proposes to continue the 2006 policy and updates how the agency will use the revised USP Model Guidelines to ensure adequate drug coverage in formularies in 2007.
More information on USP's revision of the Part D Model Guidelines is posted at<www.usp.org/healthcareInfo/mmg/phase2/index.html>.The CMS draft guidance on formulary review is posted at<http://new.cms.hhs.gov/PrescriptionDrugCovContra/03_RxContracting_FormularyGuidance.asp>.▪



