Newer Antipsychotics Show Efficacy as Depression Treatment Adjunct
The role of atypical antipsychotics has been extended to the treatment of depression. Aripiprazole, an atypical antipsychotic, was approved by the Food and Drug Administration in November for adjunctive treatment for depression.
In addition, another atypical antipsychotic, risperidone, has also shown effectiveness for patients who continue to have symptoms on antidepressants alone, according to a randomized, placebo-controlled study.
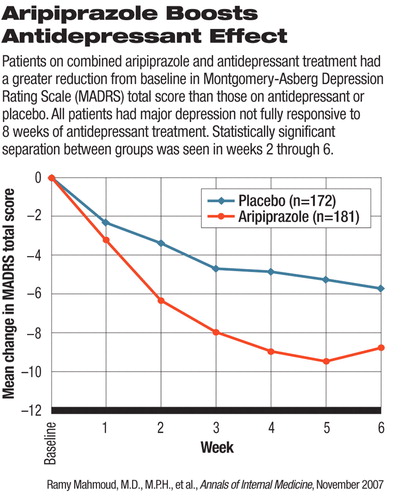
Aripiprazole, manufactured by Bristol Myers Squibb (BMS) and Otsuka Pharmaceutical, was approved on the basis of two randomized, double-blind, clinical trials in 743 adults with major depressive disorder with residual symptoms despite being treated with an antidepressant, according to a company announcement.
After all patients completed a run-in period (an eight-week period in which they took an antidepressant), those who remained symptomatic were given either aripiprazole or placebo as an add-on therapy for six weeks. The patients' clinical outcomes were rated by clinicians using the Montgomery-Asberg Depression Rating Scale (MADRS).
At the end of the study, the aripiprazole-plus-antidepressant group saw a reduction from baseline (end of the run-in period) in MADRS total score that was statistically significantly greater than that of the placebo-plus-antidepressant group. One of the two trials, which enrolled 362 patients, was published in the June Journal of Clinical Psychiatry by Robert Berman, M.D., and colleagues from BMS and Otsuka. The other trial has not been published, a BMS representative said. The daily dosage tested in both studies ranged from 2 mg to 20 mg.
The company's announcement listed the most commonly reported adverse reactions to aripiprazole, pooled from both studies, as akathisia (24 percent), restlessness (12 percent), insomnia (8 percent), constipation (5 percent), fatigue (8 percent), and blurred vision (6 percent). The mean change from baseline in weight gain was 1.7 kg (approximately 3.7 pounds) in the aripiprazole group and 0.4 kg (0.9 pounds) in the placebo group at the end of the study.
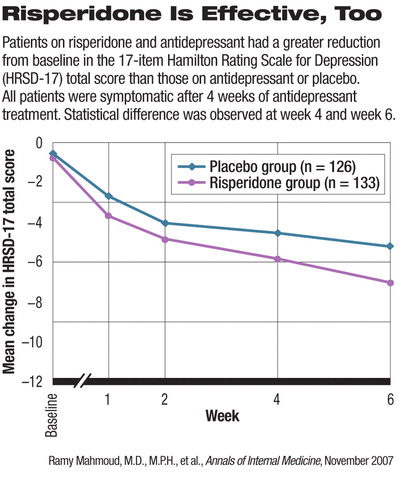
Researchers from Ortho-McNeil Janssen Scientific Affairs, led by Ramy Mahmoud, M.D., similarly found risperidone to be better than placebo as an adjunct to antidepressants. Their study was published in the November Annals of Internal Medicine.
In the randomized, double-blind trial, 268 patients who remained symptomatic despite antidepressant treatment after a four-week run-in period were randomly assigned to receive either risperidone (137 patients) or placebo (131 patients). All participants continued on their original ant idepressant as well. The risperidone dosage was started at 0.25 mg and titrated up to 1.0 mg a day in three to four weeks. Depending on response, participants might have the dose increased to a maximum of 2.0 mg.
The clinical improvement in participants, as measured by a reduction in total score on the 17-item Hamilton Rating Scale for Depression (HRSD-17) from baseline, was significantly higher in the risperidone group at week 4 and week 6. The authors also reported that significantly more patients in the risperidone group reached remission (HRSD-17 total score 7) than did patients in the placebo group. The rate of response, defined as at least 50 percent reduction in HRSD-17 total score from baseline, was also higher in the risperidone group than the placebo group. The difference between the groups in both response and remission rates was statistically significant at both week 4 and week 6.
Twenty-six patients (19 percent) in the risperidone group and 16 (12.2 percent) in the placebo group dropped out of the study. Eight patients (5.8 percent) on risperidone and three (2.3 percent) on placebo discontinued the study prematurely because of adverse events. The most frequently reported adverse events deemed treatment-related were headache (8.8 percent), dry mouth (5.1 percent), somnolence (5.1 percent), insomnia (4.4 percent), and weight gain (4.4 percent). At the end of the study, patients on risperidone gained a mean of 2.8 pounds, significantly higher than the mean of 0.3 pounds gained by the placebo group.
An abstract of “The Efficacy and Safety of Aripiprazole as Adjunctive Therapy in Major Depressive Disorder: A Multi-center, Randomized, Double-Blind, Placebo-Controlled Study” is posted at<www.psychiatrist.com/abstracts/abstracts.asp?abstract=200706/060704.htm>. An abstract of “Risperidone for Treatment-Refractory Major Depression Disorder: A Randomized Trial” is posted at<www.annals.org/cgi/content/abstract/147/9/593>.▪



