Antidepressant Data Review Finds Benefits Trump Risks
The benefits of taking antidepressants for mood and anxiety disorders far outweigh the risk of suicide for children and adolescents, according to findings from a review of more than 27 clinical trials published last month.
The findings, which appear in the April 18 Journal of the American Medical Association, prompted researchers to call on the U.S. Food and Drug Administration (FDA) to modify strict black-box warnings that currently appear on labels for antidepressants indicating an increased risk of suicidal thoughts and behaviors in youth taking them.
“Antidepressants are effective in treating disorders such as anxiety, obsessive-compulsive disorder, and depression,” said David Brent, M.D., one of the study's investigators, in an interview with Psychiatric News. While he said that they found a small risk associated with developing suicidal thoughts or behaviors in children and adolescents, he noted, “it would be much riskier not to treat them with these medications.”
Brent is a professor of psychiatry at the University of Pittsburgh School of Medicine.
He and his colleagues analyzed data from 27 randomized, placebo-controlled trials in which antidepressants were used to treat children and adolescents under the age of 19 for major depressive disorder, obsessive-compulsive disorder (OCD), or anxiety disorder. The 27 clinical trials included seven trials that were not covered in the FDA's 2004 analysis, three of which were not available at the time. Brent and his colleagues used a statistical model that differed from the one used by the FDA.
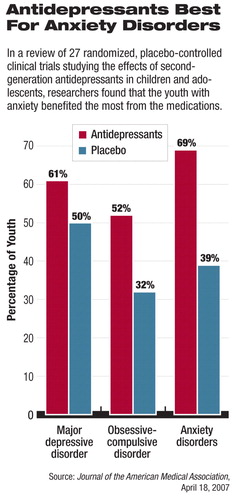
The trials in the current study took place between 1998 and 2006 and included data on roughly 5,300 patients. Fifteen of the clinical trials tested the effects of antidepressants on 3,430 subjects with depression, six of the trials tested the effects of antidepressants on 718 subjects with OCD, and another six trials tested the effects of antidepressants on 1,162 subjects with anxiety.
Antidepressants used in the trials were nefazodone, venlafa xine, mirtazepine, paroxetine, sertraline, citalopram, escitalopram, fluoxetine, and fluvoxamine.
By pooling the data across the trials, the investigators found that the antidepressants were more effective than placebo in subjects with depression, OCD, and anxiety. The drugs were most effective in children and adolescents with anxiety.
For instance, in depression trials using the Clinical Global Inventory–Improvement Scale and the Children's Depression Rating Scale–Revised, 61 percent of subjects treated with antidepressants and 50 percent of those treated with placebo responded with a 50 percent reduction in symptom severity.
In OCD trials using the Children's Yale-Brown Obsessive Compulsive Scale, 52 percent of subjects treated with antidepressants and 32 percent of those treated with placebo responded with a score reduction of 25 percent or more.
In anxiety disorder trials using the Pediatric Anxiety Rating Scale, 69 percent of subjects treated with antidepressants and 39 percent treated with placebo responded with a 50 percent reduction in scores.
Brent, pointed out, however, that “response is not the same as remission,” and that many children and adolescents with these disorders“ need a combination of psychotherapy and antidepressants.”
By pooling the data for all conditions, the investigators found a small but increased risk of treatment-emergent suicidal ideation/suicide attempts among those receiving antidepressant treatment. The statistical model they used found a smaller risk difference than the FDA found. The authors noted that overall the benefits of antidepressants appeared to be much greater than the risk from suicidal ideation/suicidal attempts.
Brent said that since youngsters with anxiety appeared to have the strongest response to antidepressants, the findings may implicate a need for early identification and treatment of anxiety symptoms, which may be a risk factor for depression.
FDA May Have Overreacted
The FDA's meta-analysis of the 24 trials included more than 4,400 children and adolescents who had received antidepressants. The agency concluded that the medications posed a twofold increased risk for suicidal behavior or ideation, prompting it to order drug manufacturers to place strongly worded black-box warnings on all antidepressants marketed in the United States (Psychiatric News, November 5, 2004).
Brent and other researchers have speculated that a jump in youth suicide rates by 18 percent between 2003 and 2004 reported in the February Pediatrics may be attributable to a steep decline in the number of prescriptions dispenses for antidepressants that began after the FDA first issued warnings about a link between antidepressants and suicidal thoughts and behavior (Psychiatric News, March 2).
Given that the findings include two large clinical trials on pediatric depression studying antidepressant effects on more than 700 children and adolescents not included in the FDA analysis, Brent said that perhaps the FDA response should be moderated.
David Mrazek, M.D., who is chair of APA's Council on Children, Adolescents, and Their Families, told Psychiatric News that the study“ provides more evidence to support the view that the benefit of treatment with antidepressants in children and adolescents clearly outweighs the minimal risk of a modest increase in suicidal ideation,” and pointed out that no children in any of the reviewed studies committed suicide while being treated with an SSRI.
Develop Better Monitoring Strategy
The study's methodological flaws may preclude a strict interpretation of its findings, experts cautioned.
APA Trustee-at-Large and child and adolescent psychiatrist Dav id Fassler, M.D., noted that while the findings confirm that antidepressants can be an effective component of treatment for children and adolescents with depression and other psychiatric disorders, the clinical trials on which the FDA and Brent reviewed reported suicidal thoughts and behaviors only as part of adverse-events reporting.
In other words, the trials were not designed to assess suicidal thoughts and behavior systematically. “Previous studies that utilized a more standardized approach to measuring suicidal ideation and intent have not demonstrated such differences in suicidality between antidepressants and placebo,” he noted.
In addition, all of the studies included in the meta-analysis were short term, but “in actual practice, many children and adolescents take these medications for a more extended period of time,” Fassler said.“ Ultimately, a more comprehensive and clinically relevant analysis of the risks and benefits will require data from multiple long-term, prospective studies.”
Darrel Regier, M.D., M.P.H., director of the American Psychiatric Institute for Research and Education and APA's Division of Research, agreed.
“What we need is for all future clinical trials to have a systematic assessment of suicidal thoughts and behaviors to enable us to get an accurate look at what the risks are,” he told Psychiatric News. “I don't think you get this from spontaneous reports [of suicidal thoughts and behavior.]”
Regier also noted that one of the problems associated with the FDA-ordered black-box warnings was that they included a recommendation for physicians to monitor patients taking the medications during seven visits within a 12-week period.
“There is no evidence that this is a cost-effective way to monitor those placed on antidepressants in a community population,” Regier said. In fact, the recommendation may discourage primary care practitioners from starting patients on antidepressants because they could not be assured that patients would return seven times in 12 weeks.
“We have an open offer to the National Institute of Mental Health and the FDA to work with APA on developing a monitoring strategy based on an evidence-based approach” such as the Patient Health Questionnaire-9 or the Quick Inventory of Depression Symptomotology–Self Report, he said.“ Improving depression monitoring is something we can do now.”
An abstract of “Clinical Response and Risk for Reported Suicidal Ideation and Suicide Attempts in Pediatric Antidepressant Treatment” is posted at<jama.ama-assn.org/cgi/content/abstract/297/15/1683>.▪



