Pharmacy Data Shed More Light on Antidepressant Trends
The Food and Drug Administration (FDA) warnings about risks of suicidal thoughts associated with antidepressants have indeed had discernible effects on the prescribing trends of these medications, especially for younger patients, a new study in the January Archives of General Psychiatry shows. Opinions differ, however, on what these trends mean.
Using prescription drug claims from the database of Medco, a large pharmacy benefit management company, Mark Olfson, M.D., M.P.H., a professor of clinical psychiatry at New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons, and two coauthors analyzed prescribing patterns for paroxetine and other antidepressants from 2002 to 2005 to investigate whether two major warnings by the FDA, announced in June 2003 and October 2004, had any measurable effect. This study was funded by the Agency for Healthcare Research and Quality and the Carmel Hill Fund.
The researchers chose three time periods for comparison: (1) from May 1, 2002 to June 19, 2003, the date that the FDA announced its suspicion that paroxetine may increase suicidal thoughts and behaviors in youth; (2) from June 20, 2003, to October 15, 2004, the date that the agency issued a black-box warning for all antidepressants on the suicide risk in children and adolescents; and (3) from October 26, 2004, to December 31, 2005.
Within each time period, according to the described methods, the database contained more than 2 million Medco members with continuous eligibility. The authors drew a 2 percent random sample from the member pool in each period. They further broke down the subjects into three age groups: youth aged 6 to 17 years, adults 18 to 64, and older adults 65 or older.
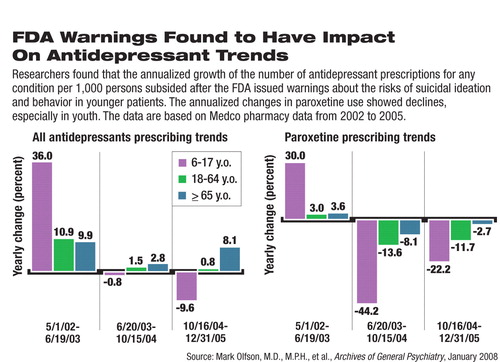
Figure. FDA Warnings Found to Have Impact on Antidepressant Trends
The actual number of patients in each category who were included in the analyses was not provided in the article.
The trends for the use of all antidepressants, regardless of what they were prescribed for, in youth changed from an increase (at an annualized rate of 36.0 percent) before June 2003 to flat (annualized rate of -0.8 percent) in the second period, to a decrease (-9.6 percent) after the black-box warning (see chart). The declining trend after October 2004, however, was not statistically significant within the period. The reverse prescribing trend was more pronounced for paroxetine, with trends changing from growth before June 2003 to a sharp decline in the second period and further decline after October 2004.
The prescribing trends in adults were less affected by the regulatory warnings. The annualized trends for all antidepressant use“ decelerated” from a pre-2003 growth rate of 10.9 percent to an essentially flat 0.8 percent after the black-box warning was issued in October 2004. The increase in prescribing for all antidepressants for older patients resumed the upward trend after the FDA's black-box warning, which defines the population at risk as pediatric patients and young adults.
The authors also analyzed new antidepressant use, defined as patients with no antidepressant use in the preceding 120 days before the first documented antidepressant claim. The annualized trends for all antidepressants for youth also changed from growth to flat, followed by a decline that was statistically nonsignificant within the final period (see chart). The sample sizes of new use and the actual percentages of use in these groups were not reported in the published study.
“When we separated out the new antidepressant use and total prescriptions,” Olfson told Psychiatric News, “the trends were largely similar.”
As the prescription declines were primarily seen in young patients under 18 and more prominent for paroxetine, the authors concluded that the FDA's warnings appeared to “have had a modest and reasonably targeted effect on antidepressant treatment patterns” and that “these changes were broadly consistent with the FDA warnings and the scientific literature.”
These conclusions contrast with those from other studies on antidepressant-use trends that were published in recent issues of the American Journal of Psychiatry (AJP). Those studies examined how the FDA's public health advisory, issued in October 2003, paralleled changing trends in antidepressant prescribing patterns in patients diagnosed with depression (Psychiatric News, June 15, September 7, and December 21, 2007). A group of researchers from the University of Colorado and Harvard Medical School found, in one of a series of studies, that significantly fewer patients between 5 and 18 years old with newly diagnosed episodes of depression were prescribed antidepressant treatment following diagnosis in the time period after the FDA advisory. These authors, along with other experts and patient advocates in psychiatry, expressed concerns that the decline in prescribing meant that many patients were not getting the pharmacologic treatment they needed.
Anne Libby, Ph.D., an assistant professor of psychiatry at the University of Colorado, told Psychiatric News, “Our studies had some methodological differences in terms of population, time-point selection, and sampling from Dr. Olfson's.” Both groups of authors collected prescription claims data from large health care insurance databases and used time-series analyses to plot trends before and after significant warnings. Both groups identified the reversal of upward trends of antidepressant prescriptions after the highly publicized regulatory actions in 2003 and 2004. Libby and her colleagues focused their data analyses on patients with a diagnosis of a new episode of depression and the medications they were prescribed, arguing that those who were already taking medications were less likely to be taken off of the drugs.
Olfson and his colleagues, meanwhile, focused primarily on total antidepressant prescriptions regardless of indication in three age groups (youth, adults, and elderly).
“Dr. Olfson's study is a cross-sectional and broader look at overall antidepressant use compared with our studies, and paroxetine in particular,” said Robert Valuck, Ph.D., an associate professor of pharmacy at the University of Colorado and a coauthor of the three AJP studies with Libby. “We tried to find out the changes in the care of patients with depression.”
In addition to decreased prescriptions for antidepressants, Libby, Valuck, and colleagues observed declining trends in the diagnosis of depression after the FDA's advisory in both young and adult patients. For those who were diagnosed with depression, they found little or no compensatory increases in nonantidepressant psychoactive medications or psychotherapy claims. Nor did they observe increased frequency in physician office visits by patients who were started on antidepressant prescriptions despite the agency's recommendation on intense monitoring for adverse events and suicidal ideation. In the Olfson study, the authors acknowledged that their data source did not allow them to assess whether or how the advisories influenced use of psychotherapy or antidepressant treatment of specific disorders.
“We did not find that there was a significant absolute decrease in the rate of antidepressant use by young people,” Olfson commented.“ Instead, we found that the rate of growth in antidepressant use significantly slowed following release of the warnings. This pattern is consistent with increased clinical caution in the use of these medications.”
Valuck and Libby, however, expressed concerns about the prescribing patterns gleaned from their own studies. “It is hard to determine whether the prescribing patterns reflect appropriate care based on these available data, which are subject to different interpretations,” said Libby. “We need more data and analyses to understand what is really going on in the context of actual patient care. We are far from finished.”
An abstract of “Effects of Food and Drug Administration Warnings on Antidepressant Use in a National Sample” is posted at<archpsyc.ama-assn.org/cgi/content/abstract/65/1/94>.▪



