How Much Influence Does Drug Industry Have on CME?
The public trusts medical professionals to provide health care services that are in the best interest of the patient and based on unbiased professional judgment. Voices within and outside of medicine, however, increasingly question how much influence pharmaceutical industry exerts on the education of physicians.
In particular, the multibillion-dollar continuing medical education (CME) industry has been criticized for being biased toward product promotion and undermining balanced and complete education to practicing physicians.

Every year pharmaceutical companies spend a large amount of money on educational grants for CME activities. The 23 largest pharmaceutical companies (based on U.S. sales) funded educational grants with budgets ranging from less than $2 million to $117 million in 2004, according to an April 2007 report by the U.S. Senate Finance Committee. Committee members Sens. Max Baucus (D-Mont.) and Charles Grassley (R-Iowa) launched an investigation in 2005 into an alleged industry practice of funding for-profit medical-communication companies that use CME activities to promote unapproved (or“ off-label”) use of new and expensive medications.
The Senate report found that from 2003 to 2005, oncology, cardiovascular disease, and “neurology/psychology” (that terminology is used in the Senate report) were the top three therapeutic areas to which drug companies allocated educational grants. In 2004, for example, 18 of the 23 largest manufacturers spent a total of $182 million on neurology/psychology. Five companies did not report data.
CME activities for physicians throughout the country are organized by a variety of groups ranging from nonprofit professional organizations (like APA) and academic medical centers to for-profit publishing or medical education companies. Providers are evaluated and accredited by the Accreditation Council for Continuing Medical Education (ACCME), whose mission is to oversee CME through “a voluntary self-regulated system for accrediting CME providers.”
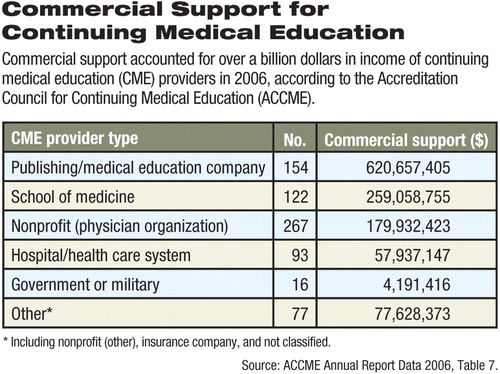
In 2006, commercial support (a term used by ACCME to classify its funding data) for CME activities accounted for $1.2 billion, while exhibits and advertising accounted for $240 million of the $2.4 billion income of all accredited CME providers, according to the organization's annual report.
For-profit medical education companies receive the most funding from the category of commercial support, but are not the only recipients of industry grants. Commercial support was a revenue source for all other types of providers including government/military providers, nonprofit and professional organizations, and medical school providers, the ACCME data showed (see chart). Many nonprofit membership organizations have a long tradition of acting as a“ firewall” between industry funding and product-specific information so that unbiased CME is provided to their healthcare professional members (see Original article: APA Enforces Strict Rules to Keep Bias out of CME for APA's policies and procedures governing industry-funded educational programs).
While pharmaceutical companies are not directly involved in creating the content and scope of educational materials, the possibility of conflict of interest in industry-funded CME activities has been criticized by academic and other advocates who believe that these educational programs, especially those created by for-profit CME providers, are thinly veiled product promotion. The Senate report stated its opinion that “it seems unlikely that this sophisticated industry would spend such large sums on an enterprise but for the expectation that the expenditures will be recouped by increased sales.”
Sponsor Bias Criticized
Intensifying criticism from academia, the media, and regulators has been directed at perceived bias in the large proportion of CME content that often favors a newly marketed product or off-label use of a product manufactured by the sponsor. The Senate report is primarily concerned about such influence on drug expenditures for Medicare and Medicaid.
The Josiah Macy Jr. Foundation recently released a summary of a report on the state of continuing education in health care professions; the report was derived from a conference conducted by the organization in November 2007 with 36 experts, mostly academics, from medicine, nursing, and education. The summary concluded that “bias, either by appearance or reality, has become woven into the very fabric of continuing education,” and that the financial responsibility of pharmaceutical companies to their shareholders and the moral responsibility of health professionals to their patients are“ fundamentally incompatible.”
Daniel Carlat, M.D., an assistant clinical professor of psychiatry at Tufts University School of Medicine and a vocal critic of industry-funded CME, agreed. “Industry-funded medical education is not education, but promotion masqueraded as education,” he told Psychiatric News.
Carlat co-chairs the Massachusetts Psychiatric Society's CME Committee. He owns and sells an accredited CME newsletter on psychiatry that indicates it receives no industry funding.
Medical Education Companies Disagree
Medical education companies, however, disagree with the criticism of industry bias. In a public response to the Macy report, the North American Association of Medical Education and Communication Companies (NAAMECC), a trade organization of medical education and communication companies, and the Coalition for Healthcare Communication, which represents marketing and publishing associations, criticized the summary. The organizations said that“ the 36 attendees did not represent the full spectrum of [continuing education] stakeholders, thus limiting the value of the recommendations and introducing serious opportunity for bias.” The CME groups' response also said that the report “ignores ongoing and increased efforts to ensure independence and decrease bias.”
“With the current accreditation system in place, FDA's guidance on industry support, and PhRMA's [Pharmaceutical Research and Manufacturers of America] code of conduct in place, there is no opportunity for sponsors to influence CME content,” said Karen Overstreet, Ed.D., past president of NAAMECC in an interview with Psychiatric News. “According to the updated ACCME accrediting standards, [industry] sponsors can no longer recommend faculty or review content of CME activities, which can be effective in eliminating the influence from commercial sponsors.”
The ACCME revised its accreditation standards several times in recent years to prevent bias, including requiring needs assessments to justify CME activities. Current ACCME rules prohibit drug or device companies to become an accredited CME provider or partner with an accredited provider to create educational materials. “The content or format of CME must promote improvements or quality in health care and not a specific proprietary business interest of a commercial interest,” one of the standards mandates.
“Of course, ultimately the CME providers are responsible for managing the conflicts of interest,” Overstreet added. ACCME reviews all providers' compliance regularly in the re-accreditation process.
In January, NAAMECC announced plans to fund a project to audit accredited CME activities randomly to quantify the presence of bias and invited other CME organizations to collaborate in the project.
Who Guards Against Influence?
The Senate report acknowledged the increased effort by the pharmaceutical and CME businesses in recent years to separate marketing from education and minimize pharmaceutical industry influence. Some pharmaceutical companies have adopted the PhRMA code of conduct for marketing, the FDA's guidance on industry-supported educational activities, Health and Human Services Office of Inspector General (HHS) compliance program guidance, and ACCME's accreditation standards for the way in which they distribute educational grants and in their involvement in CME activities. Many pharmaceutical companies and medical education and communication companies have set up “firewalls” to separate personnel involved in marketing and educational grants. Nevertheless, the committee was unsure about the extent to which these guidelines are enforced.
The report noted that because the educational activities are funded but not directly provided by manufacturers, whether CME containing off-label information can be considered promotional activity and whether the FDA has the authority to regulate such content is legally murky. The FDA's Division of Drug Marketing, Advertising, and Communications (DDMAC) is responsible for reviewing complaints about off-label promotion and refers violations to the Department of Justice and the HHS Office of Inspector General for enforcement. The Senate report concluded, “Beyond [its] guidance, the FDA does little to ensure that educational grants are used for bona fide educational purposes. Nor does the FDA have a system in place to monitor educational programs.”
The ACCME enforces its standards, including the prevention of bias, through accreditation and routine reviews. CME providers that do not comply with the policies may be put on probation or lose their accreditation. The PhRMA code and HHS compliance guidance are voluntary guidelines.
One of the recommendations in the Macy report summary called for complete elimination of commercial support of CME activities from pharmaceutical or medical-device companies. The NAAMECC opposed this recommendation in its public response: “The summary recommendations would limit free speech by both providers and supporters” and “eliminate competition, thereby limit[ing] innovation.” overstreet stressed that industry funding is an important source for a large proportion of CME activities.
Carlat supports the recommendation to prohibit industry funding for CME. He acknowledged that some may consider his stance against industry-funded CME self-serving. “I do make money from selling CME,” he commented.“ Subscribers pay me to obtain unbiased clinical information. It gives me an incentive to please the subscribers rather than my sponsor. For industry-funded CME, however, the incentive is different.”
The second part of this series will focus on the debate over industry presence in the academic training of medical students and residents.
The Senate Finance Committee's report is posted at<www.acme-assn.org/home/prb042507a.pdf>. The Macy Foundation report summary is posted at<www.acme-assn.org/home/advocacy_info/Macy_ContEd_1_7_08.pdf>. The NAAMECC's response to the Macy summary is posted at<www.naamecc.org/downloads/Macy%20Foundation%20Response.pdf>.▪



