Pain Relievers May Negate Action of SSRIs
Abstract
Do patients have to choose between being in pain or being depressed? Not necessarily, but new information will certainly raise that question for patients and their physicians. A report published online May 18 in the Proceedings of the National Academy of Sciences stated that treatment with some nonsteroidal anti-inflammatory drugs (NSAIDs) may prevent the antidepressant action of selective serotonin reuptake inhibitors (SSRIs).
The work comes from the laboratory of Paul Greengard, Ph.D., winner of the 2000 Nobel Prize in Physiology or Medicine for his discovery of how dopamine and other transmitters exert their actions in the nervous system. In the new report, Greengard and his colleagues at the Laboratory of Molecular and Cellular Neuroscience at Rockefeller University proposed a "cytokine hypothesis" of depression, saying that anti-inflammatory drugs achieve their therapeutic actions at least in part by regulation of the formation of cytokines, those small cell-signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system. The cytokines include the interleukins, lymphokines, and cell-signal molecules, such as tumor-necrosis factor and the interferons, which trigger inflammation and respond to infections.
Their hypothesis is based partly on the observation that many patients undergoing treatment involving alpha interferon or interleukin-s develop depressive symptoms and that some cytokines regulate brain norepinephrine or serotonin systems, which are linked to major depression and its treatment.
The team measured mouse brain levels of cytokines following chronic treatment with the SSRI citalopram in the presence or absence of co-treatment with ibuprofen. They identified cytokines that fell into one of three categories: those that were increased by citalopram, the effect of which was abolished by ibuprofen; those that were increased by citalopram, the effect of which was not affected by ibuprofen; and those that were not changed by either citalopram or ibuprofen. They found that ibuprofen reduced the plasma levels of citalopram and its metabolite.
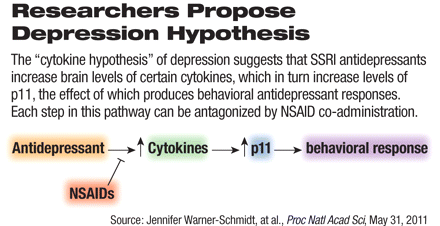
A small protein known as p11 is also implicated in the cytokine hypothesis. The actions of SSRI antidepressants are thought to increase brain levels of p11: "We have shown previously that p11 is increased in the mouse frontal cortex by multiple classes of antidepressants," wrote the researchers, who were surprised to see that co-administration of either ibuprofen or acetylsalicyclic acid (aspirin) with antidepressants for 14 days blocked the increase in p11 caused by two different SSRIs, citalopram or fluoxetine. The tricyclic antidepressant desipramine induced a small increase in p11, an increase that was not significantly affected by ibuprofen or aspirin. "Taken together, these experiments show that SSRI antidepressants increase brain levels of certain cytokines and p11, the effect of which is abolished by NSAID cotreatment," said the authors.
The team further hypothesized that two of the cytokines that were regulated by both citalopram and ibuprofen, namely, IFNγ and TNFα, were possible mediators of the inhibitory effects of NSAIDS on SSRI-induced p11 levels. Western-blot analysis revealed that both cytokines increased p11 protein in the frontal cortex compared with controls. These data support the idea that these cytokines may regulate p11 levels.
Greengard's team pointed to a well-known study in humans to back up their findings: "The antagonistic effect of anti-inflammatory agents on antidepressant-induced behaviors was confirmed by analysis of a dataset from a large-scale real-world human study, the National Institute of Mental Health's Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study.
The researchers were quick to make recommendations for practitioners: "We found that widely used anti-inflammatory drugs antagonize both biochemical and behavioral responses to selective serotonin reuptake inhibitors (SSRIs). We believe that reduced use of NSAIDs by physicians in severely depressed patients being treated with SSRIs would significantly improve positive outcomes from this major class of antidepressants. Our data indicate that clinicians should carefully balance the therapeutic benefits of anti-inflammatory agents versus the potentially negative consequences of antagonizing the therapeutic efficacy of antidepressant agents in patients suffering from depression."
In a commentary in the same issue of Proceedings of the National Academy of Sciences, Solomon Snyder, M.D., a professor of neuroscience, pharmacology, and psychiatry at the Solomon H. Snyder Department of Neuroscience at Johns Hopkins University School of Medicine, said "Greengard and colleagues report a specific molecular pathway linking cytokines and the actions of antidepressant drugs. Their findings confound established wisdom, as they imply that brain cytokines exert antidepressant actions and mediate the influences of the principal SSRI antidepressant drugs. By meandering from molecule to mental illness with implications for clinical practice, the Greengard group has reframed dialogues addressing links between the immune system and emotions."
"The clinical implications of our findings are clear," said Greengard and his colleagues. "We urge the medical community to consider these findings when designing treatment strategies for their patients that include SSRIs."
The work was supported by The Skirball Foundation, a National Institutes of Health (NIH)/National Institute of Mental Health grant, and a NIH/National Institute on Aging grant.
"Antidepressant Effects of Selective Serotonin Reuptake Inhibitors (SSRIs) Are Attenuated by Anti-Inflammatory Drugs in Mice and Humans" is posted at <www.ncbi.nlm.nih.gov/pubmed/21518864>.



