Brain Stimulation Helps in Depression, But How?
A new study on deep brain stimulation (DBS) has added to a small but growing body of evidence that suggests that it may be a promising treatment for patients with severe depression who do not respond to other available remedies.
Published online July 21 in Biological Psychiatry, the study presents results from a clinical trial conducted by Andres Lozano, M.D., Ph.D., a professor of neurosurgery at the University of Toronto, and colleagues.
Twenty patients, 11 women and nine men, with treatment-resistant depression had two small electrodes bilaterally implanted in the subcallosal cingulate gyrus (SCG) region in the brain. The surgery was performed under local anesthesia. Through batteries implanted under the skin in the chest, the electrodes continuously release electric pulses directly on the neural connections they touched.
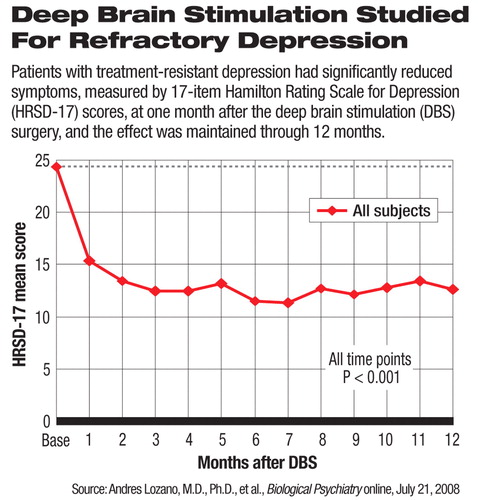
All 20 patients had previously failed to respond to at least four different courses of treatment. Eighteen of these patients were unemployed, 17 had gone through electroconvulsive therapy, and all 20 had received psychotherapy at the time of entering the study. The mean baseline score on the 17-item Hamilton Rating Scale for Depression (HRSD-17) was 24.4, suggesting moderate to severe depressive symptoms.
The severity of depression, measured by mean HRSD-17 score, significantly decreased as early as one month after the implantation and remained significantly lower than baseline through the following 12 months (see chart). At one, three, six, and 12 months after the surgery, the mean HRSD-17 scores were 15.4, 12.5, 11.8, and 12.6, respectively.
At the six-month follow-up, 60 percent of the patients achieved clinical response, which was defined as at least 50 percent reduction in the (HRSD-17) score; 35 percent met the criteria for remission, meaning that their HRSD-17 scores dropped to 7 or below. At the end of 12 months, 55 percent remain responders, and 35 percent were in remission or close to remission with a HRSD-17 score of 8 or below.
The adverse events seen in the study were not serious, the researchers noted. Three patients had wound infections. Four had headache or pain at the site of the pulse-generator implant immediately after the operation. Two had worsening mood and/or irritability. Seven patients reported no adverse effects from the operation. No patients showed long-term adverse effects from the surgery.
“This is an aggressive and costly intervention with risks similar to other brain surgeries. We needed to find out whether it is safe and whether the efficacy is sustained,” Helen Mayberg, M.D., a professor of psychiatry and neurology at Emory University and coauthor of the study, told Psychiatric News. She noted that 18 of the 20 patients had the DBS stimulation for at least one year. The other two chose not to have the device reimplanted after developing a skin infection related to the wires and returned to their baseline condition without residual adverse effects.
In addition to clinical outcomes, researchers also used positron emission tomography scans to observe directly the changes in patients' brain activities after the DBS implantation. Patients who had responded to the treatment showed“ widespread changes in metabolic activity in the limbic and cortical areas on the scans,” the authors said.
“The imaging results at three and six months after DBS showed that there were changes in not just the stimulated spot or all over the brain, but rather a specific network of brain regions,” said Mayberg. She explained that this neural network involving different regions had already been implicated in depression and recovery due to medications or psychotherapy in past imaging research.
The study was supported in part by a grant from NARSAD, and the cost of the surgeries was underwritten by Toronto Western Hospital. Mayberg emphasized that although a device company is now planning a clinical trial to further test the safety and efficacy of DBS, this published study was not funded by industry.
In addition to the SCG area, other researchers are targeting different brain regions as potential treatment for refractory depression and other disorders. For example, a study being conducted at the Cleveland Clinic is investigating the effects of DBS in the anterior limb of the internal capsule in the brain for treating depression and obsessive-compulsive disorder.
DBS is currently approved by the Food and Drug Administration for treating essential tremors, Parkinson's disease, and dystonia; the placement of the electrodes may differ depending on the indication.
“The most important questions we now have to answer are 'Who will respond to DBS?' and 'Why do they get better?'” Mayberg said.
Mayberg and her colleagues plan to conduct additional research on DBS in treating refractory depression and bipolar II depression; these efforts are independently funded by the Woodruff Fund, Stanley Medical Research Institute, and Dana Foundation, according to an Emory University press release. By learning more about the neurological networks and activities in depression and recovery, researchers hope to have a better understanding of the heterogeneity of depression and discover more effective treatments for the illness.
An abstract of “Subcallosal Cingulate Gyrus Deep Brain Stimulation for Treatment-Resistant Depression” is posted at<www.journals.elsevierhealth.com/periodicals/bps/article/S0006-3223(08)00703-8/abstract>.▪



