FDA Clears Way for Stimulation Device to Treat Opioid Withdrawal Symptoms
Abstract
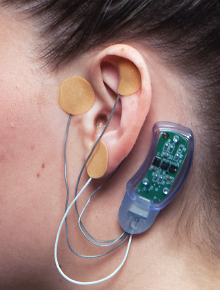
Based on principles in acupuncture, the NSS-2 Bridge device emits electrical pulses to nerves near the ear to alleviate acute withdrawal symptoms such as sweating, tremors, and gastrointestinal problems.
In November 2017, the Food and Drug Administration (FDA) approved the use of the NSS-2 Bridge neurostimulator to treat symptoms of opioid withdrawal, marking the first time that a medical device has received approval for treatment of withdrawal symptoms.

“Given the scope of the epidemic of opioid addiction, we need to find innovative new ways to help those currently addicted live lives of sobriety with the assistance of medically assisted treatment,” said FDA Commissioner Scott Gottlieb, M.D., in a press announcement about the approval. “While we continue to pursue better medicines for the treatment of opioid use disorder, we also need to look to devices that can assist in this therapy.”
The NSS-2 Bridge is a small device placed behind the patient’s ear that stimulates nearby cranial and occipital nerves (which run from the spinal cord to the scalp). These stimulations provide relief from common withdrawal symptoms during the first few days of drug abstinence, including sweating, tremors, stomach problems, insomnia, and joint pain.
This device, manufactured by Innovative Health Solutions (IHS) and available only by prescription, is strictly intended for short-term use. This limited use is insured by the fact that each individual device shuts off after 120 hours (five days), noted Tom Carrico, chief regulatory officer of IHS.
In an email to Psychiatric News, Carrico noted that in most cases, five days is long enough to clear the body of opioids and resolve the initial spate of withdrawal symptoms. After this time, the patient is reassessed by a physician for readiness to transfer to medication-assisted treatment (MAT).
Patients who are being transitioned from methadone to naltrexone may be more likely than others to receive a second prescription for NSS-2 Bridge, as methadone has a long half-life in the body. “If opioids are in someone’s system when naltrexone is administered, it may cause precipitated withdrawals, which is a serious condition,” Carrico wrote.
The FDA had previously cleared the NSS-2 Bridge in 2014 to treat acute and chronic pain. Subsequent anecdotal evidence suggested this device had some effects on withdrawal-related pains as well, leading more physicians and drug treatment centers to begin to prescribe it off label.
In March 2017, researchers at the Medical College of Wisconsin and Washington University in St. Louis published a study that compiled data from 73 patients at five separate treatment centers who had received these devices as part of their treatment for opioid use disorder. This retrospective analysis showed that after just 20 minutes of stimulation, the average clinical opiate withdrawal scale scores (considered an objective measure of withdrawal) of the participants dropped by about 63 percent; after 60 minutes, the scores dropped by 85 percent.
After five days of use, 64 of the 73 participants (88 percent) successfully transitioned to MAT.
The FDA analyzed data from this study, conducted through the de novo premarket review pathway, in its review of the NSS-2 Bridge device. This regulatory pathway fast tracks low- to moderate-risk devices that are novel and for which there is no legally marketed predicate device to which the device can be compared.
Mark George, M.D., the McCurdy Endowed Distinguished Professor at the Medical University of South Carolina and director of the university’s Brain Stimulation Laboratory, told Psychiatric News that he finds the NSS-2 Bridge to be an interesting concept.
“There is nothing new or pioneering in the technique,” he said. “But it does bring traditional acupuncture to the modern age, and makes it more user-friendly since needles are eliminated.”
(Auricular acupuncture, or auriculotherapy, is a form of acupuncture that views the ear as a gateway to the whole body.)
George added that he finds it reassuring that the device will be available by prescription only. Even though the technique is believed to be safe, noninvasive brain stimulation is a nascent field with a lot of unanswered questions, he said. ■



