CATIE's Second Phase Gives Clinicians Some Guidance
When faced with the failure of a first-choice antipsychotic medication, whether it be for lack of efficacy or intolerable side effects, both clinicians and patients alike struggle to decide which drug should be tried next. With round two results from the National Institute of Mental Health's CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness) study, the question of which antipsychotic medication to try next may now be just a bit easier to answer.
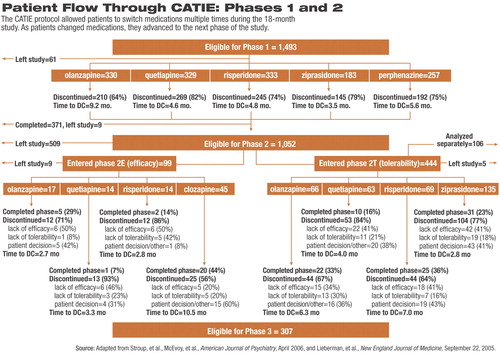
Two reports from the massive team of CATIE clinicians and researchers appear in the April American Journal of Psychiatry. Both reports detailed the findings of the study's second phase, which followed patients who discontinued taking the first medication to which they were assigned at the outset of the study. During phase 1, patients were randomly assigned to take one of the second-generation antipsychotics olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal), and ziprasidone (Geodon) or the first-generation antipsychotic perphenazine (Trilafon). Results from phase 1 of the study were reported in September 2005. Nearly three-quarters of the patients who began taking a study medication discontinued the drug prior to the 18-month endpoint of CATIE (Psychiatric News, October 21, 2005).
CATIE, with more than $61 million in funding from the National Institute of Mental Health over a 10-year commitment, enrolled nearly 1,500 patients at 57 clinical sites across the country. Jeffrey Lieberman, M.D., director of the New York State Psychiatric Institute and chair of the department of psychiatry at Columbia University College of Physicians and Surgeons, is the study's director and principal investigator.
The complex study protocol involved following patients in real-world settings through three phases of study over 18 months. The CATIE protocol specifically defined “effectiveness,” the primary outcome in all phases of the study, as the time to discontinuation of the patients' assigned medication, for any reason. In other words, the longer a patient continued to take his or her assigned medication, the more “effective” the drug was considered to be. This outcome was chosen by CATIE investigators as a proxy measure of overall patient and clinician judgment.
Of the 1,493 patients who began treatment in phase 1, 1,052 stopped taking their assigned medication prior to the study's 18-month endpoint. All patients who discontinued a phase 1 medication were eligible to continue on to either arm of phase 2 (see chart on facing page) for the remainder of the 18 months.
Splitting Pathways
The first of the new reports, from first author and CATIE co-principal investigator Joseph McEvoy, M.D., an associate professor of biological psychiatry at Duke University School of Medicine, details outcomes on patients who discontinued their phase 1 medication primarily due to lack of efficacy. In phase 2E of CATIE, these patients were randomly assigned to be treated open label with clozapine (Clozaril) or blindly assigned to receive olanzapine, quetiapine, or risperidone. Phase 2E was designed to answer the question, If a patient stops taking his or her medication due to lack of efficacy, what are the benefits of clozapine relative to the other second-generation medications as the next drug choice for that patient?
The second report, from first author and fellow CATIE co-principal investigator Scott Stroup, M.D., M.P.H., an associate professor of psychiatry at the University of North Carolina at Chapel Hill, details the results of the double-blind phase 2T arm of CATIE. Phase 2T compared the effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients who primarily discontinued taking the drug to which they had been assigned in phase 1 due to intolerability. Patients who discontinued a phase 1 medication due to lack of efficacy but were unwilling to accept the potential risks of treatment with clozapine could also continue in phase 2T. The 2T arm of CATIE was designed to compare the relative tolerability profiles of the drugs.
Clozapine Compared
During phase 2E, clozapine was clearly the most effective medication, using the CATIE definition. Nearly half of the patients in open-label treatment with clozapine continued to take the drug for the remainder of the study. For those patients who discontinued taking clozapine, the average time before they stopped taking their medication was also the longest, ranging from about seven to 16 months and averaging nearly 11 months.
Olanzapine had the second-highest proportion of phase 2E patients remaining on medication, with one-third still taking the drug at the end of the study. However, for those who did stop taking olanzapine, time to discontinuation varied widely (from two to 12 months), and the average of just under three months suggests that most patients who stopped the drug did so early on.
Patients taking risperidone in phase 2E had the third highest rate of staying on their medication through the end of the trial (14 percent); however, the mean time to discontinuation was the shortest, at under three months.
Less than 10 percent of those assigned to take quetiapine continued to take the drug through the end of the trial. The average time to discontinuation of quetiapine was just over three months.
Based on time to discontinuation, clozapine was statistically significantly better than both quetiapine and risperidone. Although the average time to discontinuation appears much better with clozapine compared with olanzapine, the difference was not statistically significant because the range of times to discontinuation were quite wide for both drugs. Fewer patients stopped taking clozapine due to lack of efficacy than any of the other three drugs, a difference that was statistically significant.
In addition, three months after entering phase 2E, patients taking clozapine had statistically significantly greater reductions in psychopathology, measured as greater reductions in scores on the Positive and Negative Syndrome Scale total score, compared with those taking quetiapine and risperidone, but not olanzapine.
Tolerability Compared
In terms of side effects during phase 2E, insomnia was most common in those taking risperidone (31 percent) and least common in those taking clozapine (4 percent). Anticholinergic symptoms were most common in those taking quetiapine (47 percent) and less common in those taking clozapine (20 percent). No significant between-drug differences were seen for metabolic measures or rate of use of hypoglycemic or lipid-controlling medications. Prolactin levels rose in patients taking risperidone and fell in those on the other three drugs.
In the group of patients taking clozapine, one of the 45 patients developed eosinophilia, and one patient developed agranulocytosis. In both cases, medication was stopped, and the patients improved.
Overall, clozapine was a significantly more effective choice, by CATIE's definition, for those patients needing to stop their assigned phase 1 medication. Surprisingly, the statistical advantages for clozapine were significant, despite the small numbers of patients in each of the comparison groups.
In phase 2T, directly comparing the four second-generation drugs without including clozapine, the treatment groups were significantly larger. Overall olanzapine and risperidone were not significantly different in terms of patients' remaining on medication through the remainder of the 18-month trial. Risperidone slightly edged out olanzapine, with ziprasidone third and quetiapine last.
In terms of time to discontinuation for any reason during phase 2T, between-drug differences were significant. Patients taking either olanzapine or risperidone continued to take their medication for nearly twice as long as patients taking either quetiapine or ziprasidone.
Overall, differences in time to discontinuation for lack of efficacy during phase 2T were statistically significant; however, differences in discontinuation due to intolerability during phase 2T were not. Yet some differences in tolerability were apparent. Patients taking olanzapine gained more weight than patients on any other drug, with those taking ziprasidone, on average, loosing weight. Olanzapine was also associated with significant increases in total cholesterol and triglycerides, while risperidone and ziprasidone were both associated with decreases in these measures.
According to the CATIE definition then, olanzapine and risperidone were more “effective” than quetiapine and ziprasidone, yet neither was as effective as clozapine.
Robert Freedman, M.D., editor in chief of the American Journal of Psychiatry, noted in a statement, “These studies are the largest, most comprehensive set of data available on the pharmacological treatment of schizophrenia. As intended, their results provide guidance to doctors, patients, and families on a logical sequence of treatments with significant information on the probability of therapeutic response and the severity of side effects at each phase.”
Darrel Regier, M.D., M.P.H., is executive director of the American Psychiatric Institute for Education and Research and director of APA's Division of Research. He added, “CATIE shows that there's no one-size-fits-all treatment. All of these medications have substantial benefits and, as with any medication, have side effects as well. It is vital that we preserve access to a full range of medications and respect physicians' clinical judgments about which medication to use and when to change.”
Regier also noted an urgent need for further research into the biological processes underlying schizophrenia and potential molecular targets for new treatments.
“Effectiveness of Clozapine Versus Olanzapine, Quetiapine, and Risperidone in Patients With Chronic Schizophrenia Who Did Not Respond to Prior Atypical Antipsychotic Treatment” is posted at<http://ajp.psychiatryonline.org/cgi/content/full/163/4/600>.“ Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic” is posted at<http://ajp.psychiatryonline.org/cgi/content/full/163/4/611>. An accompanying editorial, “Practical Treatment Information for Schizophrenia,” is posted at<http://ajp.psychiatryonline.org/cgi/content/full/163/4/563>.▪
Am J Psychiatry 2005 163 600
Am J Psychiatry 2005 163 611
Am J Psychiatry 2005 163 563



