PET Scans Reveal Action Of Methylphenidate in Brain
In what is believed to be the first direct evidence of a mechanism of action for methylphenidate, a Brookhaven National Laboratory team has produced images of significantly increased extracellular dopamine in the brain in response to therapeutic oral doses of the drug. Solidifying the mechanism of action for the drug most commonly prescribed to children with attention deficit/hyperactivity disorder (ADHD) strengthens the evidence supporting proposed theories of the pathology underlying the disorder.
Although methylphenidate has been prescribed for nearly 50 years, its mechanisms of action have been understood only poorly. A central nervous system stimulant, the drug is believed to block both the dopamine and norepinephrine transporters responsible for clearing the neurotransmitters out of the synapse after a signal has been transmitted from one neuron to the next, in the same way that an SSRI blocks the reuptake of serotonin.
 This would be beneficial in a patient with ADHD in light of recent studies that indicate that patients with the disorder have a marked increase in dopamine transporters, which are responsible for clearing the synapse of dopamine following signal transduction. This increase in the number of transporters would potentially lead to a nearly immediate increased clearing of dopamine from the nerve endings back into the neuron following its release. Researchers have hypothesized that this would result in a decrease in the intensity of the dopaminergic signal. Blocking of dopamine transport by methylphenidate could lead to stronger dopaminergic signaling, which has been tied to an increase in the “importance” of the stimulus that triggered the signal, causing an individual to pay more attention to the stimulus for a longer period.
This would be beneficial in a patient with ADHD in light of recent studies that indicate that patients with the disorder have a marked increase in dopamine transporters, which are responsible for clearing the synapse of dopamine following signal transduction. This increase in the number of transporters would potentially lead to a nearly immediate increased clearing of dopamine from the nerve endings back into the neuron following its release. Researchers have hypothesized that this would result in a decrease in the intensity of the dopaminergic signal. Blocking of dopamine transport by methylphenidate could lead to stronger dopaminergic signaling, which has been tied to an increase in the “importance” of the stimulus that triggered the signal, causing an individual to pay more attention to the stimulus for a longer period.
Although pharmacologists have hypothesized that these actions are tied to methylphenidate’s therapeutic effects in ADHD, there has been no direct evidence to date indicating a significant effect on the actual levels of neurotransmitter available for signaling at the therapeutic doses of methylphenidate commonly prescribed.
Psychiatrist Nora D. Volkow, M.D., an expert in brain imaging of drug effects and associate laboratory director for life sciences at Brookhaven in Upton, N.Y., set out to find that evidence several years ago.
“I’ve almost been obsessed about trying to understand [methylphenidate] with imaging,” Volkow said during a recent media briefing announcing the results. “As a psychiatrist, sometimes I feel embarrassed about our lack of knowledge about this drug, because this is, by far, the drug we prescribe most frequently to children.”
Volkow and her colleagues turned their attention to imaging the dopamine system, known to stimulate reward and motivation circuits when an individual experiences a pleasurable stimulus.
Volkow believed that the actual “reward” was not necessarily “pleasure” but a signal that tells the brain that “this experience is worth paying attention to.” With too much signal, Volkow explained, the experience becomes overstimulating, possibly leading to agitation. With too little signal, however, the experience leads to boredom and distraction.
Following each signal, dopamine transporters “vacuum up” the neurotransmitter from the synapse, resetting the nerve ending for the next signal. Previous work has shown that cocaine can markedly block dopamine transport, leading to an increase of as much as 50 percent in the amount of dopamine in the nerve endings, which translates into an important, “pleasurable” message.
Because of its pharmacological similarities to cocaine, Volkow’s team postulated that methylphenidate would cause a similar, but smaller, increase in dopamine. Volkow and her colleagues were rather surprised to find that the increase in dopamine was actually larger with methylphenidate than with cocaine.
The prevailing thoughts in the field in the late 1990s were that methylphenidate, which is widely metabolized, would not be absorbed into the brain in a significant enough quantity to achieve a clinically significant effect. It was further hypothesized that because the drug is absorbed slowly into the brain, rather than as a “rush” like cocaine, dopaminergic neurons would develop an adaptation response that would counteract the drug’s ability to increase dopamine.
It was not evident, according to Volkow, that therapeutic doses of the drug would increase dopamine at all, and several of Volkow’s fellow researchers thought it just might end up doing the opposite.
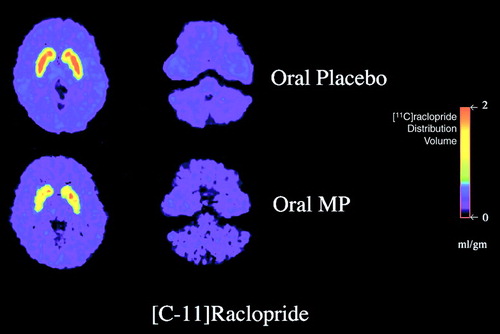
Volkow’s team used positron emission tomography (PET) to image a radioisotope (raclopride) that competed with endogenous dopamine for binding to the dopamine D2 receptor. Scans were done 60 minutes following administration of therapeutic oral doses of methylphenidate and 60 minutes after administration of a placebo control in 11 healthy male subjects.
The scans done after the subjects took methylphenidate showed a nearly 70 percent increase in extracellular dopamine in the striatum but not in the cerebellum.
“What we show [in the current study] unequivocally,” Volkow told Psychiatric News, “is that [methylphenidate] is significantly increasing extracellular dopamine levels. And you can then show that by increasing dopamine, the activity of that system is going to be amplified when you give the drug. That means that you are amplifying the system that is the one that signals salience and motivation. And so you understand why it would improve the performance of some children in school because it increases motivation and it increases the salience of the stimulation, so the child pays more attention with the drug than without it.”
Volkow and her colleagues are repeating the same protocol in a group of ADHD adult volunteers.
“We don’t have any reason to believe that the results would be any different,” she told Psychiatric News.
“Therapeutic Doses of Oral Methylphenidate Significantly Increase Extracellular Dopamine in the Human Brain” is posted on the Web at www.jneurosci.org as a “Rapid Communication.” ▪



